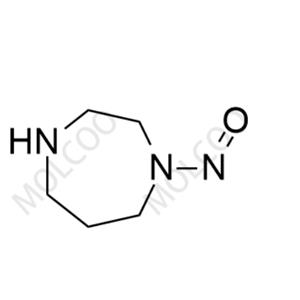
Product Details
| Product Name: Fasudil Nitroso Impurity 26 | CAS No.: 72782-14-0 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C5H11N3O |
Fasudil Nitroso Impurity 26

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Information
Product Number: F012026
English Name: Fasudil Nitroso Impurity 26
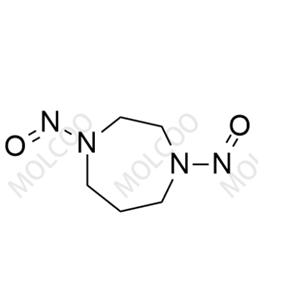
English Alias: 1-nitroso-1,4-diazepane
CAS Number: 72782-14-0
Molecular Formula: C₅H₁₁N₃O
Molecular Weight: 129.16
Advantages
Well-defined and distinct structure: Contains a 1,4-diazepane skeleton with a single nitroso (-NO) substituent. Compared with Fasudil's hexahydro-1H-1,4-diazepine core, the introduction of nitroso group results in significant differences in polarity and mass spectral response, enabling accurate identification via HPLC and LC-MS as a specific marker for impurity detection;
High stability and traceability: The conjugated structure of nitroso group and diazepane ring is stable under dark conditions. As a product of partial nitrosation of amino groups in Fasudil synthesis, it directly reflects the incompleteness of nitrosation reaction, improving the accuracy of process tracing;
High detection sensitivity: The conjugated system of nitroso group shows characteristic UV absorption (230-240nm), and combined with specific mass spectral response (m/z 130 [M+H]⁺), trace analysis can be achieved via LC-MS, compatible with detection systems for nitrogen heterocyclic drugs.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Fasudil Nitroso Impurity 26 in Fasudil APIs and formulations, ensuring residual mononitroso impurities meet quality standards;
Synthesis process optimization: Optimizing reagent ratios (e.g., nitrite dosage) and reaction time of nitrosation by monitoring impurity content to reduce mononitroso by-product formation;
Stability evaluation: Assessing the oxidative deterioration trend of Fasudil formulations during storage to support the development of appropriate storage conditions (e.g., light protection, airtight packaging).
Background Description
Research Status
Detection method optimization: Using UPLC-MS/MS with optimized parameters based on characteristic fragment ions of nitroso groups (e.g., NO⁺) to achieve trace detection (detection limits up to ppb level);
Nitrosation kinetics: Studying impurity formation rates under varying pH and reaction time to clarify key factors driving partial amino nitrosation in Fasudil;
Toxicological assessment: Evaluating genotoxic potential via in vitro assays (e.g., micronucleus test) to establish scientifically based impurity limits;
Inhibition strategies: Developing nitrosation-inhibiting additives (e.g., ascorbic acid) to minimize impurity formation in Fasudil production and storage.
Product Information
Product Number: F012026
English Name: Fasudil Nitroso Impurity 26
English Alias: 1-nitroso-1,4-diazepane
CAS Number: 72782-14-0
Molecular Formula: C₅H₁₁N₃O
Molecular Weight: 129.16
Advantages
Well-defined and distinct structure: Contains a 1,4-diazepane skeleton with a single nitroso (-NO) substituent. Compared with Fasudil's hexahydro-1H-1,4-diazepine core, the introduction of nitroso group results in significant differences in polarity and mass spectral response, enabling accurate identification via HPLC and LC-MS as a specific marker for impurity detection;
High stability and traceability: The conjugated structure of nitroso group and diazepane ring is stable under dark conditions. As a product of partial nitrosation of amino groups in Fasudil synthesis, it directly reflects the incompleteness of nitrosation reaction, improving the accuracy of process tracing;
High detection sensitivity: The conjugated system of nitroso group shows characteristic UV absorption (230-240nm), and combined with specific mass spectral response (m/z 130 [M+H]⁺), trace analysis can be achieved via LC-MS, compatible with detection systems for nitrogen heterocyclic drugs.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Fasudil Nitroso Impurity 26 in Fasudil APIs and formulations, ensuring residual mononitroso impurities meet quality standards;
Synthesis process optimization: Optimizing reagent ratios (e.g., nitrite dosage) and reaction time of nitrosation by monitoring impurity content to reduce mononitroso by-product formation;
Stability evaluation: Assessing the oxidative deterioration trend of Fasudil formulations during storage to support the development of appropriate storage conditions (e.g., light protection, airtight packaging).
Background Description
Research Status
Detection method optimization: Using UPLC-MS/MS with optimized parameters based on characteristic fragment ions of nitroso groups (e.g., NO⁺) to achieve trace detection (detection limits up to ppb level);
Nitrosation kinetics: Studying impurity formation rates under varying pH and reaction time to clarify key factors driving partial amino nitrosation in Fasudil;
Toxicological assessment: Evaluating genotoxic potential via in vitro assays (e.g., micronucleus test) to establish scientifically based impurity limits;
Inhibition strategies: Developing nitrosation-inhibiting additives (e.g., ascorbic acid) to minimize impurity formation in Fasudil production and storage.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-23 | |
| $33.00/50mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-10-26 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 |








 China
China