
Product Details
| Product Name: Rivaroxaban Nitroso Impurity | Min. Order: 1mg |
| Purity: >95% HPLC | Supply Ability: 10000 |
| Release date: 2025/07/31 | |
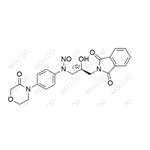
| Molecular Formula:: C21H20N4O6 | Molecular Weight:: 424.41 |
| Appearance: Pale yellow soild | Storage: 2-8°C Refrigerator |
| Product Catalog: | R005109 |
| CAS No.: | |
| Product Name: | Rivaroxaban Nitroso Impurity |
| Purity: | >95% HPLC |
| Synonyms: | (S)-N-(3-(1,3-dioxoisoindolin-2-yl)-2-hydroxypropyl)-N-(4-(3-oxomorpholino)phenyl)nitrous amide |
| Molecular Formula: | C21H20N4O6 |
| Mol. Weight: | 424.41 |
| Appearance: | Pale yellow soild |
| Storage: | 2-8°C Refrigerator |
| Contact: | WhatsAPP: +86 17320513646 E-mail: anna@molcoo.com |
| Note: | We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS. This product is intended for laboratory use only! |
| Background: | Rivaroxaban is a widely used anticoagulant drug. The nitroso impurity in rivaroxaban is of concern due to the potential safety risks associated with nitroso compounds. Nitroso impurities may form during the synthesis, storage, or degradation of rivaroxaban. These impurities are known for their potential mutagenicity and carcinogenicity, which could have significant implications for the safety and quality of rivaroxaban - containing medications. Understanding and controlling this impurity is crucial to ensure the safe use of rivaroxaban. |
Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-20 | |
| $8.00/1kg |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2024-04-25 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-08-07 |
INQUIRY








 China
China