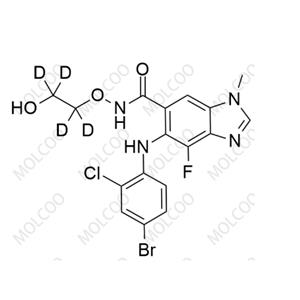
Product Details
| Product Name: Selumetinib-d4 | CAS No.: 2364350-70-7 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C5H11N3O |
Selumetinib-d4;2364350-70-7

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Information
Product Number: S041029
English Name: Selumetinib-d4
English Alias: Selumetinib-d4
CAS Number: 2364350-70-7
Molecular Formula: C₁₇H₁₁D₄BrClFN₄O₃
Molecular Weight: 461.71
Advantages
Stable structure with clear labeling: Contains 4 deuterium (D) atoms at specific positions, with a molecular weight 4 units higher than Selumetinib. It can be accurately distinguished via mass spectrometry (MS) by characteristic ion peaks (m/z 462 [M+H]⁺), serving as a stable internal standard for quantitative analysis;
Low isotopic effect: C-D bonds at deuterated sites (e.g., methyl or methylene groups) are more stable than C-H bonds, minimizing hydrogen-deuterium exchange during in vitro/in vivo metabolism and ensuring accurate quantification;
High detection sensitivity: Combined with LC-MS/MS, the specific isotopic response of deuterated analogs enables precise quantification of Selumetinib in biological samples (e.g., plasma, tissues) at trace levels (ng/mL), reducing matrix interference.
Applications
Pharmacokinetic studies: Used as an internal standard in LC-MS/MS methods to quantify Selumetinib concentrations in blood, tissues, and excreta, supporting dose optimization and metabolic pathway analysis;
Bioavailability assessment: Comparing in vivo exposure of Selumetinib across formulations (tablets, capsules) using deuterated internal standard data to evaluate bioavailability differences;
Metabolite identification: Assisting in the characterization of oxidative or hydrolytic metabolites by comparing retention times and fragmentation patterns with the deuterated standard.
Background Description
Research Status
Method validation: Developing LC-MS/MS assays with Selumetinib-d4 as internal standard, achieving quantification limits as low as 0.1 ng/mL for clinical sample analysis;
Isotope effect evaluation: Confirming minimal impact of deuteration on Selumetinib’s metabolism via in vitro liver microsome studies, validating internal standard suitability;
Clinical assay standardization: Implementing deuterated internal standard-based methods in multi-center trials to reduce inter-laboratory variability in pharmacokinetic data;
Formulation development: Using quantitative results with Selumetinib-d4 to optimize drug solubility and dissolution rate in oral formulations, improving bioavailability.
Product Information
Product Number: S041029
English Name: Selumetinib-d4
English Alias: Selumetinib-d4
CAS Number: 2364350-70-7
Molecular Formula: C₁₇H₁₁D₄BrClFN₄O₃
Molecular Weight: 461.71
Advantages
Stable structure with clear labeling: Contains 4 deuterium (D) atoms at specific positions, with a molecular weight 4 units higher than Selumetinib. It can be accurately distinguished via mass spectrometry (MS) by characteristic ion peaks (m/z 462 [M+H]⁺), serving as a stable internal standard for quantitative analysis;
Low isotopic effect: C-D bonds at deuterated sites (e.g., methyl or methylene groups) are more stable than C-H bonds, minimizing hydrogen-deuterium exchange during in vitro/in vivo metabolism and ensuring accurate quantification;
High detection sensitivity: Combined with LC-MS/MS, the specific isotopic response of deuterated analogs enables precise quantification of Selumetinib in biological samples (e.g., plasma, tissues) at trace levels (ng/mL), reducing matrix interference.
Applications
Pharmacokinetic studies: Used as an internal standard in LC-MS/MS methods to quantify Selumetinib concentrations in blood, tissues, and excreta, supporting dose optimization and metabolic pathway analysis;
Bioavailability assessment: Comparing in vivo exposure of Selumetinib across formulations (tablets, capsules) using deuterated internal standard data to evaluate bioavailability differences;
Metabolite identification: Assisting in the characterization of oxidative or hydrolytic metabolites by comparing retention times and fragmentation patterns with the deuterated standard.
Background Description
Research Status
Method validation: Developing LC-MS/MS assays with Selumetinib-d4 as internal standard, achieving quantification limits as low as 0.1 ng/mL for clinical sample analysis;
Isotope effect evaluation: Confirming minimal impact of deuteration on Selumetinib’s metabolism via in vitro liver microsome studies, validating internal standard suitability;
Clinical assay standardization: Implementing deuterated internal standard-based methods in multi-center trials to reduce inter-laboratory variability in pharmacokinetic data;
Formulation development: Using quantitative results with Selumetinib-d4 to optimize drug solubility and dissolution rate in oral formulations, improving bioavailability.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1KG |
VIP2Y
|
Shaanxi Xianhe Biotech Co., Ltd
|
2025-05-06 | |
| $0.00/10g |
Cangzhou Kangrui Pharma Tech Co. Ltd.,
|
2024-10-17 | ||
| $47.00/25mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-11-04 |


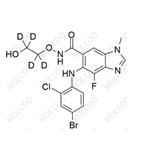








 China
China