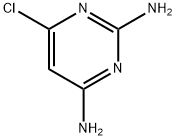
Synthesis, Reactivity, and Functional Transformations of 4-Chloro-2,6-diaminopyrimidine
Dec 22,2025
Reactivity Enhancement through Amino Group Acetylation
4-chloro-2,6-diaminopyrimidine is a crucial intermediate in the synthesis of important chemotherapeutic antibacterials. This compound is reported to give nucleophilic chlorine substitution only under quite drastic conditions: as an example, the displacement by pure piperidine requires 3 h at 100 °C. The acetylation of both amino groups in 4-chloro-2,6-diaminopyrimidine increases the rate of the chlorine substitution so drastically to allow room-temperature piperidinolysis in few minutes. [1]

Kinetic Parameters of Piperidino-Dechlorination in DMSO
The second-order rate constants kA for the piperidino-dechlorination of 4-chloro-2,6-diaminopyrimidine have been determined from the corresponding pseudo-first-order rate constants, kψ, measured in DMSO at 21.0 °C by the UV spectrophotometric procedure. The second-order rate coefficients of the less reactive substrates have been obtained as extrapolated values from Arrhenius plots of kA values, calculated through the pseudo-first-order-type relationship, kψ = kA[P] (where [P] is the amine concentration), from the kψ measured at higher temperatures (kA = 1.36 x 10-5), and the extrapolated values at 21℃ are kA = 1.36 x 10-5 L mol-1 min-1. For the reaction of piperidine with 4-Chloro-2,6-diaminopyrimidine 2,4, ∆H* = 11.9 ± 0.3 kcal/mol and ∆S* = —40 eu. In 4-chloro-2,6-diaminopyrimidine, the monosubstitution of both the amino groups with electronwithdrawing groups, such as acetyls, increases noticeably the reactivity of this substrate, while N(3)-oxidation gives an analogous but feeble effect. [1]
Synthetic Routes to 4-Chloro-2,6-diaminopyrimidine and Their Relative Efficiency
As part of a continuing program of cancer chemotherapy, a number of pyrimidine derivatives have been synthesized in these laboratories for biological evaluation and as precursors of condensed pyrimidine ring systems. Recently the synthesis of various substituted pyrimidines using 4-amino-6-chloro-2-methylthiopyrimidine as a versatile starting material was reported. The key intermediate utilized in this investigation was 4-chloro-2,6-diaminopyrimidine. This compound was prepared by two different approaches, one involving chlorination of a diaminohydroxypyrimidine, the other requiring animation of an aminodichloro compound. [2]
For the first route, 2,4-diamino-6-hydroxypyrimidine was prepared by condensation of guanidine with ethyl cyanoacetate. When the usual Traube conditions were followed, 2,4-diamino-6-hydroxypyrimidine was obtained in 45% yield along with an 8-12% yield of cyanoacetylguanidine. The latter compound, originally reported by Traube, is formed by reaction of the ester group of ethyl cyanoacetate as evidenced by nitrile absorption in the infrared. The total yield of 2,4-diamino-6-hydroxypyrimidine could be increased to 58-64% by conversion of the uncyclized by-product into 2,4-diamino-6-hydroxypyrimidine in refluxing 1 N sodium hydroxide. However, the preparation of 2,4-diamino-6-hydroxypyrimidine based upon the procedure of VanAllan, further modified by precipitation of the product at pH 8, was found to be superior to the Traube method and afforded 2,4-diamino-6-hydroxypyrimidine in 80-85% yield. Chlorination of 2,4-diamino-6-hydroxypyrimidine with phosphorus oxychloride by modification of the procedure of Roth, et al., afforded 4-chloro-2,6-diaminopyrimidine in 62-71% yield. [2]
The alternate route to 4-chloro-2,6-diaminopyrimidine, via monoamination of 2-amino-4,6-dichloropyrimidine, involved first the preparation of 2-amino-4,6-dihydroxypyrimidine from guanidine and diethyl malonate according to the method of Traube. Refluxing 2-amino-4,6-dihydroxypyrimidine with phosphorus oxychloride in the absence of dimethylaniline8 led to 2-amino-4,6-dichloropyrimidine in 50% yield after purification by either recrystallization or sublimation. 2-amino-4,6-dichloropyrimidine was then monoaminated with ethanolic ammonia at 160℃ following the procedure of Büttner. This route afforded 4-chloro-2,6-diaminopyrimidine in 20% over-all yield in three steps from acyclic materials, whereas the first described sequence led to a 57% over-all yield of 2,4-diamino-6-hydroxypyrimidine in two steps and obviated the pressure reaction. [2]
Controlled Nitration Pathways and Product Selectivity
Nitration of 4-chloro-2,6-diaminopyrimidine has been studied under a variety of reaction conditions. It was found that when equal volumes of concentrated H2SO4 and fuming HNO3 were used, the product was 2-amino-4nitramino-6-chIoropyrimidine rather than 2,4-diamino-5-nitro-6-chloropyrimidine. The desired derivative 2,4-diamino-5-nitro-6-chloropyrimidine could be obtained in 61% yield when a large excess of concentrated H2SO4 (5:1 relative to the amount of HNO3) was used. The optimum temperature for this reaction is 20-35℃. [3]
References:
[1] Maltese, M. (1995). Relative Reactivity in Piperidino-Dechlorination of 2, 4-Diamino-6-chloropyrimidine and 2, 4-Diamino-6-chloropyrimidine N (3)-Oxide and Their Acetylamino Analogs. The Journal of Organic Chemistry, 60(8), 2436-2442.
[2] Israel, M., Protopapa, H. K., Schlein, H. N., & Modest, E. J. (1964). Pyrimidine Derivatives. VI. 2, 4, 5-Triamino-6-chloro-and-6-mercaptopyrimidine and Related Compounds1, 2. Journal of Medicinal Chemistry, 7(6), 792-799.
[3] O'Brien, D. E., Cheng, C. C., & Pfleiderer, W. (1966). Pyrimidines. XVIII. 2, 4-Diamino-5-nitro-6-arylaminopyrimidines. Nitration Study of 2, 4-Diamino-6-chloropyrimidine and a Literature Correction1. Journal of Medicinal Chemistry, 9(4), 573-575.
- Related articles
- Related Qustion
- 4-Chloro-2,6-diaminopyrimidine: properties, applications and safety Oct 12, 2023
4-Chloro-2,6-diaminopyrimidine is a white crystalline solid with potential environmental applications and associated safety risks.
- What is 4-Chloro-2,6-diaminopyrimidine? Jan 16, 2020
4-Chloro-2,6-diaminopyrimidine(DACP) is a member of pyrimidines and an organohalogen compound. It is a white crystal-like solid.
2-(2-chlorophenyl)cyclohexanone has been documented in the literature for its application in the synthesis of ketamine.....
Dec 22,2025APISelenium is an essential micronutrient for human body, this article will introduce the benefits and safety of Se-Methylselenocysteine.....
Dec 22,2025Amino Acids and Derivatives4-Chloro-2,6-diaminopyrimidine
156-83-2You may like
4-Chloro-2,6-diaminopyrimidine manufacturers
- 4-Chloro-2,6-diaminopyrimidine
-
- 2025-12-22
- CAS:156-83-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 4-Chloro-2,6-diaminopyrimidine
-

- $5.00 / 25kg
- 2025-12-22
- CAS:156-83-2
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 1000mt/year
- 4-Chloro-2,6-diaminopyrimidine
-
- $0.00 / 25KG
- 2025-12-01
- CAS:156-83-2
- Min. Order: 1KG
- Purity: 98
- Supply Ability: 10000KGS