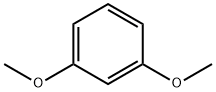
2,4-Dimethoxybenzoic acid synthesis
- Product Name:2,4-Dimethoxybenzoic acid
- CAS Number:91-52-1
- Molecular formula:C9H10O4
- Molecular Weight:182.17

17715-69-4
304 suppliers
$9.00/10g
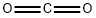
124-38-9
134 suppliers
$175.00/23402

91-52-1
234 suppliers
$10.60/1gm:
Yield:91-52-1 91%
Reaction Conditions:
Stage #1:1-Bromo-2,4-dimethoxybenzene with n-butyllithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2:carbon dioxide in tetrahydrofuran at -78; for 0.75 h;
Steps:
4.1.4. 2,4-Dimethoxybenzoic acid (9)22
n-BuLi (2.5 M in THF, 3.3 mL, 8.3 mmol) was added drop wise to a stirred solution of 1-bromo-2,4-dimethoxybenzene (7) (1.5 g, 6.9 mmol) in THF (15 mL) at -78 °C under N2 atmosphere. After 30 min, CO2 gas was passed through the solution during 45 min of time and the mixture was allowed to warm up to room temperature. THF was removed in vacuum and the mixture was treated with saturated NaHCO3 solution (40 mL). The water layer was washed with ethyl acetate (2*20 mL) and then acidified with conc. HCl. The mixture was extracted with ethyl acetate (2*75 mL). The organic layer was washed with water (2*30 mL), brine (30 mL), dried (Na2SO4), filtered and concentrated. Recrystallization from ethyl acetate produced 9 (1.15 g, 91%) as white solid. Rf 0.3 (1:1 ethyl acetate:hexane); mp 103-105 °C (lit.22 107-109 °C); 1H NMR (CDCl3, 500MHz): δ 8.10 (d, 1H, J=9.0Hz, C6-H), 6.61 (dd, 1H, J=9.0, 2.0Hz, C5-H), 6.50 (d, 1H, J=2.0Hz, C3-H), 4.01 (s, 3H, OMe), 3.86 (s, 3H, OMe); 13C NMR (CDCl3, 125MHz): δ 179.9 (C), 165.2 (C), 160.2 (C), 135.2 (CH), 110.1 (C), 106.2 (CH), 98.5 (CH), 56.4 (CH3), 55.7 (CH3).
References:
Pahari, Pallab;Saikia, Ujwal Pratim;Das, Trinath Prasad;Damodaran, Chendil;Rohr, Jürgen [Tetrahedron,2016,vol. 72,# 23,p. 3324 - 3334] Location in patent:supporting information

613-45-6
499 suppliers
$10.00/5g

91-52-1
234 suppliers
$10.60/1gm:

2150-41-6
104 suppliers
$18.00/1g

91-52-1
234 suppliers
$10.60/1gm:

31874-34-7
54 suppliers
$45.00/250mg

91-52-1
234 suppliers
$10.60/1gm: