
2,5‐ bis(triMethylstannyl)th ieno[3,2‐b]thiophene synthesis
- Product Name:2,5‐ bis(triMethylstannyl)th ieno[3,2‐b]thiophene
- CAS Number:469912-82-1
- Molecular formula:C12H20S2Sn2
- Molecular Weight:465.84
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
278 suppliers
$8.00/250mg
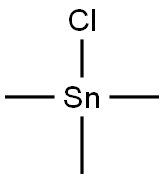
1066-45-1
160 suppliers
$26.04/2g
![2,5‐
bis(triMethylstannyl)th
ieno[3,2‐b]thiophene](/CAS/20180906/GIF/469912-82-1.gif)
469912-82-1
119 suppliers
$35.00/100mg
Yield:469912-82-1 68%
Reaction Conditions:
Stage #1: thieno[3,2-b]thiophenewith n-butyllithium in tetrahydrofuran at -78 - 20; for 4 h;
Stage #2: trimethylstannyl chloride in tetrahydrofuran at -78 - 20; for 16.5 h;
Steps:
1 Preparation of compound 8a
Commercially available thieno[3,2-b]thiophene (4 g, 32 mmol) in THF (100 mL) was cooled to -78 °C and n-butyllithium solution (1.6 M, 42 mL, 66 mmol) was added drop wise over 60 minutes using a dropping funnel. The reaction mixture was gradually warmed to room temperature and stirred for 3 hrs. The resultant suspension was again cooled to -78 °C and a solution of trimethyltin chloride (13.15 g, 66 mmol) in THF (50 mL) was added drop wise over 30 minutes using a dropping funnel. The resultant mixture was gradually warmed to room tem-perature and stirred for 16 hrs. The reaction mixture was quenched with water (150 mL) and extracted with Et.20 (2 x 100 mL). Combined organic layers were washed with brine and concentrated to give brown solids, which were triturated with ethanol (4 x 20 mL). The solids were collected by filtration and washed thoroughly with ethanol (2 x 20 mL) to yield compound 8a as a white solid (10 g, 68%), which was used directly in the next step without further purification. H NMR (400 MHz, CDCI3) δ 7.26 (s, 2H), 0.37 (s, 18H).
References:
WO2014/87300,2014,A1 Location in patent:Page/Page column 23; 24

1066-45-1
160 suppliers
$26.04/2g
![2,5‐
bis(triMethylstannyl)th
ieno[3,2‐b]thiophene](/CAS/20180906/GIF/469912-82-1.gif)
469912-82-1
119 suppliers
$35.00/100mg
![Thieno[3,2-b]thiophene-2-carboxylic acid ethyl ester](/CAS2/GIF/201004-08-2.gif)
201004-08-2
42 suppliers
$77.76/250mgs:
![2,5‐
bis(triMethylstannyl)th
ieno[3,2‐b]thiophene](/CAS/20180906/GIF/469912-82-1.gif)
469912-82-1
119 suppliers
$35.00/100mg
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
278 suppliers
$8.00/250mg
![2,5‐
bis(triMethylstannyl)th
ieno[3,2‐b]thiophene](/CAS/20180906/GIF/469912-82-1.gif)
469912-82-1
119 suppliers
$35.00/100mg

930-96-1
243 suppliers
$11.00/1g
![2,5‐
bis(triMethylstannyl)th
ieno[3,2‐b]thiophene](/CAS/20180906/GIF/469912-82-1.gif)
469912-82-1
119 suppliers
$35.00/100mg