
2-THIENYL-2-DIMETHYLAMINOETHYL KETONE HCL synthesis
- Product Name:2-THIENYL-2-DIMETHYLAMINOETHYL KETONE HCL
- CAS Number:5424-47-5
- Molecular formula:C9H14ClNOS
- Molecular Weight:219.73

88-15-3
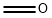
50-00-0

506-59-2

5424-47-5
2-Acetylthiophene (128 g, 1.00 mol), dimethylamine hydrochloride (90 g, 1.10 mol), paraformaldehyde (41 g, 1.40 mol), concentrated hydrochloric acid (5 mL), and isopropanol (500 mL) were mixed and heated and refluxed for 6 hours according to the method reported by Robertson [1]. Upon completion of the reaction, the reaction solution was cooled to 0 °C and stirring was continued for 1 hour. Subsequently, the reaction mixture was filtered, the solid product was washed with cold isopropanol and finally dried at 50 °C for 16 h to give 3-dimethylamino-1-(thiophen-2-yl)-propan-1-one hydrochloride (2a).

88-15-3
609 suppliers
$5.00/10g
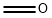
50-00-0
896 suppliers
$10.00/25g

506-59-2
556 suppliers
$5.00/5 g

5424-47-5
207 suppliers
$9.00/5g
Yield: 94%
Reaction Conditions:
with hydrogenchloride in water;isopropyl alcohol for 6 h;Reflux;
Steps:
3.1 3-(dimethylamino)-1-(2-thienyl)-1-propanone hydrochloride 2a:
Following a protocol defined by Robertson [1], a mixture of 2-acetylthiophene (128g, 1000 mmol), dimethylamine hydrochloride (90 g,1100 mmol), paraformaldehyde (41 g, 1400 mmol), concentrated hydrochloric acid(5 mL) and isopropanol (500 mL) was refluxed for 6 h. After the completion ofthe reaction, the obtained solution was cooled to 0 oC and stirredfor 1 h more. The slurry was then filtered, and the solid was washed with coldisopropanol, dried for 16 h at 50 oC to afford 2a.
References:
Chen, Xiang;Liu, Zhi-Qiang;Lin, Chao-Ping;Zheng, Yu-Guo [Bioorganic Chemistry,2016,vol. 65,p. 82 - 89] Location in patent:supporting information
