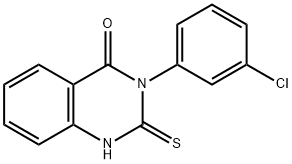
3-(3-CHLORO-PHENYL)-2-MERCAPTO-3H-QUINAZOLIN-4-ONE synthesis
- Product Name:3-(3-CHLORO-PHENYL)-2-MERCAPTO-3H-QUINAZOLIN-4-ONE
- CAS Number:1028-38-2
- Molecular formula:C14H9ClN2OS
- Molecular Weight:288.75
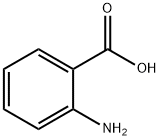
118-92-3
0 suppliers
$23.00/250g

2392-68-9
100 suppliers
$23.00/1g

1028-38-2
17 suppliers
$45.00/50mg
Yield:1028-38-2 70%
Reaction Conditions:
with triethylamineReflux;
Steps:
General synthetic procedure for the synthesisof compounds (1-8)
General procedure: Phenyl isothiocyanate derivative (1 mmol) and anthranilicacid (1 mmol) were added in absolute ethanol (10 ml) intoa 100-ml round-bottomed flask. Few drops of triethylaminewere added in the reaction mixture and refluxed for 2-3 h.Reaction progress was checked periodically by TLC analysis.On completion, precipitates were appeared in the reactionflask which were collected via filtration and washedwith distilled water and then hexane to remove trace impurities.Crude products were crystallized from ethanol toobtained purified products.
References:
Wali, Hayat;Anwar, Ayaz;Shamim, Shahbaz;Khan, Khalid Mohammed;Mahdavi, Mohammad;Salar, Uzma;Larijani, Bagher;Perveen, Shahnaz;Taha, Muhammad;Faramarzi, Mohammad Ali [Journal of the Iranian Chemical Society,2021,vol. 18,# 8,p. 2017 - 2034]
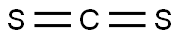
75-15-0
272 suppliers
$40.00/100g
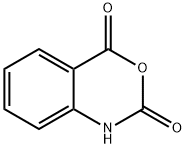
118-48-9
446 suppliers
$5.00/5G
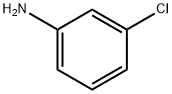
108-42-9
480 suppliers
$10.00/5g

1028-38-2
17 suppliers
$45.00/50mg

877-81-6
0 suppliers
inquiry

1028-38-2
17 suppliers
$45.00/50mg

108-42-9
480 suppliers
$10.00/5g

1028-38-2
17 suppliers
$45.00/50mg
