
3-AMINO-2-BROMO-BENZOIC ACID METHYL ESTER synthesis
- Product Name:3-AMINO-2-BROMO-BENZOIC ACID METHYL ESTER
- CAS Number:106896-48-4
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
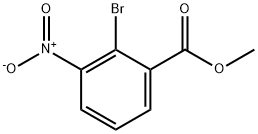
5337-09-7

106896-48-4
Methyl 2-bromo-3-nitrobenzoate (12.9 g, 49.5 mmol) was used as feedstock and prepared according to the method described by Webber ES et al. (Patent Application No. WO 01/16136 A2). The feedstock was refluxed with SnCl2 (42 g, 223 mmol) in a mixed solution of methanol (225 mL, 0.2 M) and H2O (5.3 g, 243 mmol) for 2 hours. After completion of the reaction, it was cooled to room temperature and diatomaceous earth (20 g) and dichloromethane (1 L) were added, followed by the slow addition of 3 N aqueous sodium hydroxide solution (150 mL) under vigorous stirring. The reaction mixture was filtered and the organic phase was washed with saturated aqueous sodium chloride solution. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to remove all volatiles to afford the intermediate methyl 3-amino-2-bromobenzoate (11.4 g) in 98% yield.1H-NMR (d6-DMSO) δ: 7.12 (dd, 1H, J = 8.1, 7.5 Hz), 6.93 (dd, 1H, J = 8.1, 1.6 Hz), 6.80 ( dd, 1H, J = 7.4, 1.6 Hz), 5.57 (s, 2H), 3.81 (s, 3H).

5337-09-7
105 suppliers
$20.00/250mg

106896-48-4
92 suppliers
$20.00/100mg
Yield: 98%
Reaction Conditions:
with tin(ll) chloride in methanol;water for 2 h;Heating / reflux;
Steps:
5.1 Step 1. Preparation OF 3-AMINO-2-BROMO-BENZOIC acid methyl ester 5 (a)
2-Bromo-3-nitro-benzoic acid methyl ester (12.9 g, 49.5 MMOL) (prepared from 2-amino-3-nitro- benzoic acid as described by Webber E. S. et AL., see patent application number WO 01/16136 A2) and SNCI2 (42 g, 223 MMOL) were REFLUXED in methanol (225 mL, 0.2 M) and H20 (5.3 G, 243 MMOL) for 2 hours. After cooling at ambient temperature, diatomaceous earth (20 G) and DICHLOROMETHANE (1 L) were added followed with 3N aqueous sodium hydroxide (150 mL) with vigorous stirring. The mixture was filtered and the organic phase was washed with saturated aqueous sodium chloride. The organic solution was dried over sodium sulfate, filtered and all volatiles were removed under reduced pressure to afford Intermediate 5 (a) (11.4 G) in 98% yield. 1H-NMR (d6-DMSO) : ô 7. 12 (dd, 1H, J = 8. 1,7. 5 Hz), 6.93 (dd, 1H, J = 8. 1,1. 6 Hz), 6.80 (dd, 1H, J = 7.4, 1.6 Hz), 5.57 (s, 2H), 3.81 (s, 3H).
References:
PFIZER INC. WO2004/63198, 2004, A1 Location in patent:Page 77

570-24-1
363 suppliers
$6.00/10g

106896-48-4
92 suppliers
$20.00/100mg

41085-43-2
199 suppliers
$6.00/1g

106896-48-4
92 suppliers
$20.00/100mg

4518-10-9
196 suppliers
$12.80/1g

6942-37-6
91 suppliers
$19.00/250mg

106896-48-4
92 suppliers
$20.00/100mg

4518-10-9
196 suppliers
$12.80/1g

6942-37-6
91 suppliers
$19.00/250mg
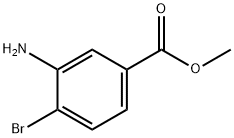
46064-79-3
149 suppliers
$10.00/1g

106896-48-4
92 suppliers
$20.00/100mg