
3-Aminobenzeneboronic acid synthesis
- Product Name:3-Aminobenzeneboronic acid
- CAS Number:30418-59-8
- Molecular formula:C6H8BNO2
- Molecular Weight:136.94
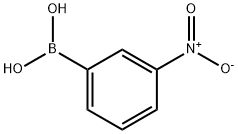
13331-27-6

30418-59-8
General method: 3-nitrophenylboronic acid (1 eq.), hexafluoroisopropanol (HFIP, 10 eq.) and iron powder (5 eq.) were added to the reaction tube and mixed. Subsequently, 2N aqueous hydrochloric acid solution was added dropwise to the reaction mixture. The reaction mixture was stirred at room temperature for 30 minutes and then neutralized with saturated aqueous sodium bicarbonate (NaHCO3). The organic layer was extracted three times with saturated aqueous sodium bicarbonate solution and combined. The organic layer was subsequently washed with brine, dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure. Finally, the crude product was purified by silica gel column chromatography to afford the target compound 3-aminophenylboronic acid.

13331-27-6
355 suppliers
$5.00/1g

30418-59-8
262 suppliers
$6.00/1g
Yield:30418-59-8 83%
Reaction Conditions:
Stage #1:m-nitrobenzene boronic acid with hydrogenchloride;1,1,1,3',3',3'-hexafluoro-propanol;iron in water at 20; for 0.5 h;
Stage #2: with sodium hydrogencarbonate in waterchemoselective reaction;
Steps:
2. General Procedure for the Reduction of Nitro Compounds
General procedure: The nitro compound (1 equiv), HFIP (10 equiv), Fe powder (5 equiv) were mixed in a tube. Then 2 N HCl aqueous solutions was added to the reaction mixture. After stirring at room temperature for 30 min, the reaction mixture was neutralized with sat. NaHCO3 (aq.) and extracted with EtOAc three times. The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting crude product was then purified by column chromatography on silica gel to furnish the desired amine product.
References:
Chen, Xu-Ling;Ai, Bai-Ru;Dong, Yu;Zhang, Xiao-Mei;Wang, Ji-Yu [Tetrahedron Letters,2017,vol. 58,# 37,p. 3646 - 3649] Location in patent:supporting information

585-79-5
30 suppliers
$15.00/1g
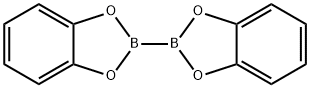
13826-27-2
142 suppliers
$5.00/250mg

30418-59-8
262 suppliers
$6.00/1g

1160186-73-1
21 suppliers
$85.00/500mg
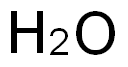
7732-18-5
506 suppliers
$12.70/100ml

30418-59-8
262 suppliers
$6.00/1g
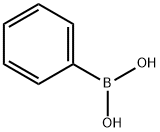
98-80-6
746 suppliers
$5.00/5g

30418-59-8
262 suppliers
$6.00/1g

591-19-5
335 suppliers
$10.00/10g

30418-59-8
262 suppliers
$6.00/1g