
3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:34761-09-6
- Molecular formula:C11H11NO2S
- Molecular Weight:221.28

394-47-8

623-51-8
![3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/34761-09-6.gif)
34761-09-6
The general procedure for the synthesis of ethyl 3-aminobenzo[B]thiophene-2-acetate from 2-fluorobenzonitrile and ethyl mercaptoacetate is as follows: Method 1: Synthesis of 4-aminobenzothiophene from 3,4-dipyrimidine 1A. Ethyl 3-amino-carboxylate 2-fluorobenzonitrile (8.25 mmol), diisopropylethylamine (8.25 mmol), and ethyl 2-mercaptoacetate (8.25 mmol) were dissolved in anhydrous N,N-dimethylformamide (5 mL) and stirred for 30 min at room temperature. Potassium carbonate (8.25 mmol) was then added and the reaction mixture was heated to 80 °C and maintained for 20 hours. Upon completion of the reaction, the reaction was quenched by the addition of ice water, the precipitate was collected by filtration and washed with water to afford the target product carboxylate as a white solid in 92% yield. LCMS retention time 7.43 min, [M + H]+ 222; 1H-NMR (CDCl3) δ 7.43 (1H, d, J = 8.1 Hz), 7.65 (1H, d, J = 8.1 Hz), 7.49-7.44 (1H, m), 7.39-7.34 (1H, m), 4.36 (2H, q, J = 7.1 Hz), 1.40 (3H, t, J = 7.1 Hz).

394-47-8
481 suppliers
$5.00/5g

623-51-8
242 suppliers
$7.00/25g
![3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/34761-09-6.gif)
34761-09-6
65 suppliers
$20.00/250mg
Yield:34761-09-6 92%
Reaction Conditions:
Stage #1: 2-fluorobenzonitrile;ethyl 2-sulfanylacetatewith N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: with potassium carbonate in N,N-dimethyl-formamide at 80; for 20 h;
Steps:
1.1A
Method 1 : 4-Aminobenzothienof3,2-dlpyrimdineSynthesis 1AEthyl 3-amin -carboxylate2-Fluorobenzonitrile (8.25 mmol), diisopropylethylamine (8.25 mmol) and ethyl2-mercaptoacetate (8.25 mmol) in anhydrous Ν,Ν-dimethylformamide (5 mL) was allowed to stir for 30 min at room temperature. Potassium carbonate (8.25 mmol) was added and the solution was heated to 80°C for 20 h. Ice-water was added and the resulting precipitate was filtered off, washing with water to obtain the carboxylate as a white solid (92%).LCMS rt 7.43, M+H 222; 1H-NMR (CDCI3) δ 7.43 (1H, d, J 8.1 Hz), 7.65 (1H, d, J 8.1 Hz), 7.49-7.44 (1H, m), 7.39-7.34 (1H, m), 4.36 (2H, q, J 7.1 Hz), 1.40 (3H, t, J 7.1 Hz).
References:
WO2012/131297,2012,A1 Location in patent:Page/Page column 85

7716-66-7
277 suppliers
$32.00/5g

105-53-3
789 suppliers
$5.00/25g
![3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/34761-09-6.gif)
34761-09-6
65 suppliers
$20.00/250mg

874-05-5
202 suppliers
$15.00/1g

623-51-8
242 suppliers
$7.00/25g
![3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/34761-09-6.gif)
34761-09-6
65 suppliers
$20.00/250mg

623-51-8
242 suppliers
$7.00/25g

612-24-8
149 suppliers
$20.00/5g
![3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/34761-09-6.gif)
34761-09-6
65 suppliers
$20.00/250mg
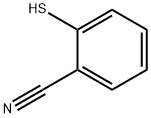
34761-11-0
21 suppliers
inquiry

105-36-2
351 suppliers
$5.00/5G
![3-AMINO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/34761-09-6.gif)
34761-09-6
65 suppliers
$20.00/250mg