
4,4'-DDT synthesis
- Product Name:4,4'-DDT
- CAS Number:50-29-3
- Molecular formula:C14H9Cl5
- Molecular Weight:354.49

79-37-8
484 suppliers
$17.67/10gm:

5471-77-2
88 suppliers
$20.00/100mg

50-29-3
106 suppliers
$25.00/1mg
Yield:-
Reaction Conditions:
with SiO2 in dichloromethane;ethyl acetate;N,N-dimethyl-formamide;acetonitrile;Petroleum ether
Steps:
165.4 Step-4:
Step-4:
Compound 4
To a stirred solution of 3-ethoxy-2,2-dimethyl-3-oxopropanoic acid (10 g, 62.4 mmol) in dichloromethane (100 ml) at 0° C. was added oxalyl chloride (6.4 g, 75.6 mmol) followed by two drops DMF.
The reaction mixture was stirred at room temperature for 2 h and then concentrated under vacuum to obtain brown oil.
This was then added dropwise into a solution of 3 (15.5 g, 51.3 mmol) and DIPEA (36 ml, 206.7 mmol) in acetonitrile (100 ml) at 0° C. and stirred at room temperature for 16 h.
The reaction mixture was completely concentrated under vacuum to obtain a residue.
One more similar batch of reaction was carried out in parallel and both the residues were taken together in ethyl acetate.
The ethyl acetate layer was washed with 1.5 N HCl, 10% NaHCO3 solution and brine, dried over anhydrous Na2SO4, filtered and concentrated.
The crude was purified using column chromatography (SiO2, 15-20% ethyl acetate in petroleum ether) to yield compound 4 as white solid (25 g, 59.7%).
References:
Petter, Russell C.;Jewell, Charles F.;Lee, Kwangho;Medikonda, Aravind Prasad;Niu, Deqiang;Qiao, Lixin;Singh, Juswinder;Zhu, Zhendong US2011/269244, 2011, A1 Location in patent:Page/Page column

75-87-6
190 suppliers
inquiry

108-90-7
685 suppliers
$10.00/25G

50-29-3
106 suppliers
$25.00/1mg

789-02-6
81 suppliers
$49.00/25mg

75-87-6
190 suppliers
inquiry

108-90-7
685 suppliers
$10.00/25G
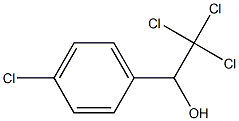
5333-82-4
5 suppliers
inquiry

50-29-3
106 suppliers
$25.00/1mg

75-87-6
190 suppliers
inquiry

108-90-7
685 suppliers
$10.00/25G

50-29-3
106 suppliers
$25.00/1mg

4714-30-1
0 suppliers
inquiry
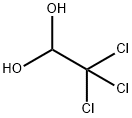
302-17-0
454 suppliers
$27.00/1mg

108-90-7
685 suppliers
$10.00/25G

50-29-3
106 suppliers
$25.00/1mg