
4-METHYLTHIOPHENE-3-CARBOXYLIC ACID synthesis
- Product Name:4-METHYLTHIOPHENE-3-CARBOXYLIC ACID
- CAS Number:78071-30-4
- Molecular formula:C6H6O2S
- Molecular Weight:142.18

30318-99-1
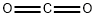
124-38-9

78071-30-4
At -78 °C, n-butyllithium (n-BuLi, 1.6 M hexane solution, 14.6 mL, 23.3 mmol) was slowly added dropwise to a stirred solution of 3-bromo-4-methylthiophene (2.7 g, 15.6 mmol) in tetrahydrofuran (THF, 35 mL). After the dropwise addition was completed, the reaction mixture was kept stirred at -78 °C for 15 minutes, followed by continued stirring for 30 minutes. Next, carbon dioxide gas (CO2) was passed into the reaction mixture for 10 minutes and stirring was continued at the same temperature for 20 minutes. Upon completion of the reaction, the mixture was slowly warmed to 0 °C and the reaction was quenched with 1 M aqueous sodium hydroxide (NaOH) solution (60 mL), followed by washing with ethyl acetate (EtOAc, 2 x 50 mL). The aqueous phase was acidified to pH about 5 and extracted with dichloromethane (DCM, 2 × 50 mL). The organic layers were combined, washed sequentially with water (100 mL) and saturated saline (100 mL), dried over anhydrous sodium sulfate (Na2SO4), and concentrated under reduced pressure. Finally, the residue was purified by column chromatography (silica gel as stationary phase, 8% methanol/dichloromethane as eluent) to afford the target product 4-methylthiophene-3-carboxylic acid (1.5 g, 70% yield) as a white solid.

30318-99-1
213 suppliers
$6.00/1g
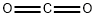
124-38-9
134 suppliers
$175.00/23402

78071-30-4
105 suppliers
$34.00/100mg
Yield: 70%
Reaction Conditions:
Stage #1:3-bromo-4-methylthiophene with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2:carbon dioxide in tetrahydrofuran;hexane at -78; for 0.5 h;
Steps:
A56.1 Step 1 : 4-methylthiophene-3-carboxylic acid (A56-1)
To a stirred solution of 3-bromo-4-methylthiophene (2.7 g, 15.6 mmol) in THF (35 mL) was added n-BuLi (1.6 M in hexane, 14.6 mL, 23.3 mmol) at -78 °C dropwise over a period of 15 min and the mixture was stirred at -78 °C for 30 min. The C02 (gaseous) was passed through the reaction mixture for 10 min and the mixture was stirred at the same temperature for 20 min. Thereafter, the reaction mixture was warmed to 0 °C, quenched with aqueous 1 M NaOH (60 mL) and washed with EtOAc (2 x 50 mL). The aqueous layer was acidified to pH ~ 5 and extracted with DCM (2 x 50 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, 8% MeOH/DCM as eluent) to provide compound A56-1 (1.5 g^ 70%) as a white solid
References:
TEIJIN PHARMA LIMITED;AMGEN INC.;BECK, Hilary Plake;BOOKER, Shon Keith;BREGMAN, Howard;CEE, Victor J.;CHAKKA, Nagasree;CUSHING, Timothy D.;EPSTEIN, Oleg;FOX, Brian M.;GEUNS-MEYER, Stephanie;HAO, Xiaolin;HIBIYA, Kenta;HIRATA, Jun;HUA, Zihao;HUMAN, Jason;KAKUDA, Shinji;LOPEZ, Patricia;NAKAJIMA, Ryota;OKADA, Kazuhisa;OLSON, Steven H.;OONO, Hiroyuki;PENNINGTON, Lewis D.;SASAKI, Kosuke;SHIMADA, Keiko;SHIN, Youngsook;WHITE, Ryan D.;WURZ, Ryan P.;YI, Shuyan;ZHENG, Xiao Mei WO2015/129926, 2015, A1 Location in patent:Page/Page column 78

73229-39-7
46 suppliers
inquiry

78071-30-4
105 suppliers
$34.00/100mg

43088-42-2
177 suppliers
$5.00/250mg

78071-30-4
105 suppliers
$34.00/100mg

16494-41-0
4 suppliers
inquiry

78071-30-4
105 suppliers
$34.00/100mg