
5-Bromo-1H-benzimidazole synthesis
- Product Name:5-Bromo-1H-benzimidazole
- CAS Number:4887-88-1
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03

1575-37-7
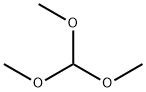
149-73-5

4887-88-1
Step 87a: Synthesis of tert-butyl 5-bromo-1H-benzo[d]imidazole-1-carboxylate (compound 0601-187) To a solution of 4-bromo-1,2-benzenediamine (3 g, 16 mmol) in N,N-dimethylformamide (DMF, 22 mL) was added trimethyl orthoformate (44 mL), which was subsequently concentrated. Concentrated hydrochloric acid (1.5 mL) was added and the reaction mixture was stirred at room temperature for 1 hour. Upon completion of the reaction, the mixture was diluted with deionized water (200 mL) and the pH was adjusted to 7 with saturated aqueous sodium bicarbonate. the reaction mixture was extracted with ethyl acetate (200 mL) and the organic layer was dried with anhydrous sodium sulfate and concentrated under reduced pressure to give 5-bromo-1H-benzo[d]imidazole (3.25 g, 100%) as an off-white solid. lc-MS (mass spectrum): m/z 197 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 7.33 (t, J = 8.8 Hz, 1H), 7.55 (dd, J1 = 7.6 Hz, J2 = 40 Hz, 1H), 7.79 (d, J = 47.2 Hz, 1H), 8.26 (s, 1H), 12.61 (d, J = 25.6 Hz, 1H ).

1575-37-7
370 suppliers
$10.00/1g

149-73-5
545 suppliers
$12.00/5g

4887-88-1
198 suppliers
$5.00/100mg
Yield:4887-88-1 100%
Reaction Conditions:
Stage #1:4-Bromo-benzene-1,2-diamine;trimethyl orthoformate with hydrogenchloride in water;N,N-dimethyl-formamide at 20; for 1 h;
Stage #2: with sodium hydrogencarbonate in water;N,N-dimethyl-formamide; pH=7
Steps:
87.87a
Step 87a: 7¾rt-butyl 5-bromo-lH-benzo[d]imidazole-l-carboxylate (Compound 0601-187)To a solution of 4-bromobenzene-l,2-diamine (3 g, 16 mmol) in DMF (22 mL) were added trimethyl orthoformate (44 mL) and cone. HC1 (1.5 mL) and the mixture was stirred at room temperature for 1 h. The mixture was diluted with water (200 mL) and adjusted to pH7 with saturated aqueous NaHC03, extract with ethyl acetate (200 mL). The organic layer was dried over Na2S04, concentrated to give 5-bromo-lH-benzo[d]imidazole (3.25 g, 100%) as an off-white solid. LCMS: 197 [M+l]+. 1H NMR (400 MHz, DMSO-<¾) δ 7.33 (t, J= 8.8 Hz, 1H), 7.55 (dd, J; = 7.6 Hz, J= 40 Hz, 1H), 7.79 (d, J= 47.2 Hz, 1H), 8.26 (s, 1H), 12.61 (d, J= 25.6 Hz, 1H).
References:
CURIS, INC.;BAO, Rudi;LAI, Chengjung;QIAN, Changgeng WO2011/130628, 2011, A1 Location in patent:Page/Page column 228
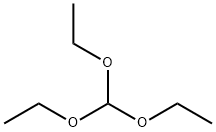
122-51-0
514 suppliers
$10.00/5ml

1575-37-7
370 suppliers
$10.00/1g

4887-88-1
198 suppliers
$5.00/100mg
64-18-6
1133 suppliers
$22.13/250ML

1575-37-7
370 suppliers
$10.00/1g

4887-88-1
198 suppliers
$5.00/100mg

875-51-4
306 suppliers
$5.00/1g

1758-73-2
474 suppliers
$18.00/25g

4887-88-1
198 suppliers
$5.00/100mg

68468-39-3
48 suppliers
$197.00/1g

4887-88-1
198 suppliers
$5.00/100mg