
5-BROMO-2-METHYL-1H-BENZIMIDAZOLE synthesis
- Product Name:5-BROMO-2-METHYL-1H-BENZIMIDAZOLE
- CAS Number:1964-77-8
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06

78-39-7

1575-37-7

1964-77-8
GENERAL METHOD: A mixture of 4-bromophthalimide (1.0 mmol), triethyl orthoacetate (1.2 mmol) and ZrCl4 (0.1 mmol) in 10 mL of methanol was stirred at room temperature for 3 hours. Upon completion of the reaction, the progress was monitored by TLC and the solvent was removed by concentration under reduced pressure. The crude product was purified by silica gel column chromatography with an eluent ratio of petroleum ether/ethyl acetate (4:1 to 1:1, v/v) to afford the target compound 5-bromo-2-methyl-1H-benzo[D]imidazole.
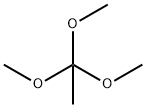
1445-45-0
356 suppliers
$10.00/5mL

875-51-4
305 suppliers
$5.00/1g

1964-77-8
135 suppliers
$6.00/250mg
Yield: 90%
Reaction Conditions:
with indium;acetic acid in ethyl acetate for 4 h;Reflux;Inert atmosphere;
Steps:
4.2. General procedure for the indium-mediated reductive reaction of 2-nitroanilines or 1,2-dinitroarenes with trimethyl orthoesters to benzimidazoles
General procedure: 2-Nitroaniline derivative (1 mmol) was added to a mixture of indium powder (574 mg, 5.0 mmol for 2-nitroaniline, 918 mg 8.0 mmol for 1,2-dinitroarene), and acetic acid (0.572 mL, 10 mmol) in ethyl acetate (2 mL), followed by the addition of trimethyl orthoester (2.0 mmol) in ethyl acetate (3 mL for 2-nitroaniline; 8 mL for 1,2-dinitroarene). The reaction mixture was stirred at reflux under a nitrogen atmosphere. After the reaction was completed, the reaction mixture was diluted with ethyl acetate (30 mL), filtered through Celite, poured into 10% NaHCO3 (30 mL), and then extracted with ethyl acetate (30 mL×3). The combined organic extracts were dried over MgSO4, filtered, and concentrated. The residue was eluted with ethyl acetate/hexane (v/v=10/90) for 2-phenylbenzimidazole derivatives or methanol/dichloromethane (v/v=1/99) for 2-methylbenzimidazole derivatives through a silica gel column to give the corresponding benzimidazoles. The structures of the benzimidazoles were characterized by 1H NMR, 13C NMR, FTIR, and GC-MS, and were mostly known compounds. HRMS data were reported in addition for unknown compounds.
References:
Kim, Jaeho;Kim, Jihye;Lee, Hyunseung;Lee, Byung Min;Kim, Byeong Hyo [Tetrahedron,2011,vol. 67,# 41,p. 8027 - 8033] Location in patent:experimental part

78-39-7
375 suppliers
$9.00/1g

1575-37-7
371 suppliers
$10.00/1g

1964-77-8
135 suppliers
$6.00/250mg

78191-00-1
287 suppliers
$6.00/5g

1575-37-7
371 suppliers
$10.00/1g

1964-77-8
135 suppliers
$6.00/250mg
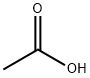
64-19-7
1629 suppliers
$10.00/25ML

1575-37-7
371 suppliers
$10.00/1g

1964-77-8
135 suppliers
$6.00/250mg

615-15-6
320 suppliers
$9.00/1g

1964-77-8
135 suppliers
$6.00/250mg