
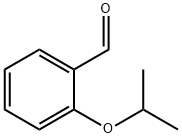
5-BROMO-2-ISOPROPOXYBENZALDEHYDE synthesis
- Product Name:5-BROMO-2-ISOPROPOXYBENZALDEHYDE
- CAS Number:138505-25-6
- Molecular formula:C10H11BrO2
- Molecular Weight:243.1

75-30-9
1761-61-1

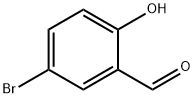
138505-25-6
Step A: Synthesis of 5-bromo-2-isopropoxybenzaldehyde (AX). To a suspension of dimethylformamide (DMF) containing potassium carbonate (34.4 g, 249 mmol) and cesium carbonate (16.2 g, 50 mmol) were sequentially added 5-bromosalicylaldehyde (25.0 g, 124 mmol) and 2-iodopropane (25.0 mL, 249 mmol). The reaction mixture was stirred at room temperature overnight, followed by heating to 70 °C and continued stirring for 4 hours. Upon completion of the reaction, the volatile solvent was removed by rotary evaporation. The residue was dissolved and partitioned between methyl tertiary butyl ether (MTBE) and water. The aqueous layer was further extracted with MTBE and all organic phases were combined and washed sequentially with water, sodium hydroxide solution and saturated brine. The organic layer was dried over anhydrous magnesium sulfate and concentrated to dryness under reduced pressure to afford 5-bromo-2-isopropoxybenzaldehyde (AX, 30.0 g) as a light yellow oil in 99% yield.1H NMR (CDCl3, 400 MHz): δ 1.40 (d, J = 6.3 Hz, 6H), 4.65 (sept, J = 6.0 Hz, 1H), 6.89 (d, J = 9.0 Hz, 1H), 7.59 (dd, J = 9.0 and 2.7 Hz, 1H), 7.91 (d, J = 2.7 Hz, 1H), 10.39 (s, 1H).

75-30-9
278 suppliers
$15.00/1g

1761-61-1
388 suppliers
$5.00/5g

138505-25-6
52 suppliers
$58.00/250mg
Yield:138505-25-6 99%
Reaction Conditions:
with potassium carbonate;caesium carbonate in N-methyl-acetamide;
Steps:
29.A Synthesis of DArPhin Catalysts
Step A: Synthesis of 5-bromo-2-isopropoxybenzaldehyde AX. To a suspension of potassium carbonate (34.4 g, 249 mmol) and cesium carbonate (16.2 g, 50 mmol) in dimethylformamide were added 5-bromosalicaldehyde (25.0 g, 124 mmol) and 2-iodopropane (25.0 mL, 249 mmol). The suspension was stirred at room temperature overnight, then at 70° C. for 4 hrs. The volatiles were removed, and the residue was partitioned between methyl t-butylether and water. The aqueous layer was extracted with methyl t-butylether and the combined organic phases were washed with water, sodium hydroxide, and brine, and then dried over magnesium sulfate. Concentration to dryness afforded compound AX (30.0 g) as a pale yellow oil in 99% yield. 1H NMR (CDCl3, 400 MHz): δ 1.40 (d, J=6.3 Hz, 6H), 4.65 (sept., J=6.0 Hz, 1H), 6.89 (d, J=9.0 Hz, 1H), 7.59 (dd, J=9.0 and 2.7 Hz, 1H), 7.91 (d, J=2.7 Hz, 1H), 10.39 (s, 1H).
References:
US2009/202480,2009,A1
22921-58-0
81 suppliers
$42.00/500mg

138505-25-6
52 suppliers
$58.00/250mg

515832-52-7
48 suppliers
$43.30/250mg

138505-25-6
52 suppliers
$58.00/250mg

75-26-3
474 suppliers
$10.00/10g

1761-61-1
388 suppliers
$5.00/5g

138505-25-6
52 suppliers
$58.00/250mg

40530-18-5
185 suppliers
$15.00/1g

138505-25-6
52 suppliers
$58.00/250mg