
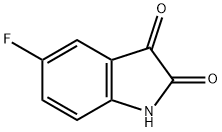
5-Fluoro-3-indazolecarboxylic acid synthesis
- Product Name:5-Fluoro-3-indazolecarboxylic acid
- CAS Number:1077-96-9
- Molecular formula:C8H5FN2O2
- Molecular Weight:180.14
443-69-6

1077-96-9
The general procedure for the synthesis of 5-fluoroindazole-3-carboxylic acid from 5-fluoroindigo is as follows: first, sodium hydroxide (1.24 g, 0.031 mol, 1.03 eq.) was dissolved in water (19 mL) and heated to 50 °C, then 5-fluoroindigo (5 g, 0.03 mol, 1 eq.) was added. 5 minutes later, the resulting deep red solution was cooled to 0 °C, followed by the slow addition of a pre-cooled sodium nitrite (2.07 g, 0.03 mol, 1 eq.) solution in water (10 mL). Immediately thereafter, the cooled 95% sulfuric acid (3.12 mL, 0.06 mol, 1.95 eq.) water (48 mL) solution was added rapidly, controlling the rate of addition to ensure that the temperature did not exceed 4°C. A small amount of ether may be added at the appropriate time to minimize foaming during mixing. After the addition was complete, stirring was continued at less than 4°C for 1 hour. Subsequently, a 35% hydrochloric acid (24 mL) solution of pre-cooled stannous chloride (16.24 g, 0.072 mol, 2.4 eq.) was added and the mixture was stirred for 16 hours. After completion of the reaction, the brown solution was filtered and the resulting brown solid was washed with water to give a final light brown solid product (2.73 g, 51% yield). The NMR hydrogen spectral (MeOH-d4) data of the product were as follows: δH 7.25 (1H, td, J = 9.10 Hz and 2.14 Hz, ArH), 7.60 (1H, dd, J = 9.10 Hz and 4.28 Hz, ArH), 7.74 (1H, dd, J = 9.10 Hz and 2.10 Hz, ArH); LC-MS-EI showed the molecular ion peak m/z 183.1 (MH+, 100).

443-69-6
390 suppliers
$5.00/1g

1077-96-9
134 suppliers
$52.00/250mg
Yield: 51%
Reaction Conditions:
Stage #1:5-fluoro-1H-indole-2,3-dione with sodium hydroxide in water at 50; for 0.0833333 h;
Stage #2: with sodium nitrite in water at 0 - 4; for 1 h;
Stage #3: with hydrogenchloride in water for 16 h;
Steps:
4
To a solution of NaOH (1.24 g, 0.031 mol, 1.03 equiv.) in water (19 mL) heated at 50CC was added 5-Fluoroisatin (5 g, 0.03 mol, 1 equiv.). After 5 minutes, the dark- red solution was cooled to 0°C and a cooled solution of sodium nitrite (2.07 g, 0.03 mol, I equiv.) in water (10 mL) was added slowly, followed by a cooled solution of (95%) (3.12 mL, 0.06 mol , 1.95 equiv.) in water (48 mL). The rate of addition was rapid such that the temperature never rose above 4°C. To reduce the foaming which occurred throughout the period of stirring, a few millilitres of ether were added when necessary. After the end of addition, the cooled solution was stirred for one hour, maintaining the temperature below 4 °C at all times. Then a cooled solution of SnC 1 (16.24 g, 0.072 mol, 2.4 equiv.) in HCl (35%) (24 mL) was added and the mixture was stirred for 16 h. The brown solution was filtrated and the resultion brown solid was washed with water to give a light brown solid (2.73 g, 51%); δΗ (MeOH-£/6) 7.25 (1H, td, J 9.10 Hz and J2.14 Hz, ArH), 7.60 (1H, dd J 9.10 Hz and 4.28 Hz, ArH), 7.74 (1H, dd J 9.10 Hz and 2.10 Hz, ArH); LC-MS-EI 183.1 (MH+, 100)
References:
UNIVERSITY COLLEGE LONDON;POSADA, Cristina, Garcia;SELWOOD, David;GARTHWAITE, John;BAKER, David;CLUTTERBUCK, Lisa WO2011/61469, 2011, A1 Location in patent:Page/Page column 34-35