
6-BROMO-2H-1,4-BENZOXAZIN-3(4H)-ONE synthesis
- Product Name:6-BROMO-2H-1,4-BENZOXAZIN-3(4H)-ONE
- CAS Number:24036-52-0
- Molecular formula:C8H6BrNO2
- Molecular Weight:228.04

40925-68-6
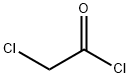
79-04-9

24036-52-0
General procedure for the synthesis of 6-bromo-2H-1,4-benzoxazin-3(4H)-one from 2-amino-4-bromophenol and chloroacetyl chloride: triethylamine (TEA, 4.06 g, 40 mmol) was added to a tetrahydrofuran (THF, 150 mL) solution of 2-amino-4-bromophenol (5 g, 27 mmol). Subsequently, chloroacetyl chloride (3.33 g, 30 mmol) was added in batches at 0 °C. After 20 min of reaction, the mixture was continued to be stirred for 2 h at room temperature. The reaction mixture was again cooled to 0 °C and sodium hydride (NaH, 60% dispersed in mineral oil, 2.2 g, 54 mmol) was added in batches. After stirring at 0 °C for 20 min, the reaction mixture was brought to room temperature and stirring was continued for 2 h. The reaction mixture was then quenched with water. After completion of the reaction, the reaction was quenched with water. The solvent was removed by distillation under reduced pressure and the resulting residue was diluted with water. The precipitate was collected by filtration, washed with water and dried under vacuum to afford 6-bromo-2H-1,4-benzoxazin-3(4H)-one (5.5 g, 89% yield).

7693-52-9
236 suppliers
$5.00/1g

105-36-2
353 suppliers
$5.00/5 g

24036-52-0
190 suppliers
$9.00/250mg
Yield: 85%
Reaction Conditions:
Stage #1:4-bromo-2-nitrophenol;ethyl bromoacetate with potassium carbonate in acetonitrile for 2 h;Reflux;
Stage #2: with iron;acetic acid at 80; for 2 h;
Steps:
1.A Step A
Compound 7-1 (25g, 0.12mol) was dissolved in CH3CN (250ml), add K2CO3 (42g, 0.23mol) and ethyl 2-bromoacetate (30g, 0.18mol). The mixture was stirred at reflux for 2h, filtered, and the filtrate was concentrated to give crude product. The crude product was dissolved in acetic acid (250ml), was added Fe (20g, 0.36mol). The mixture was stirred at 80 2h, then poured into water, extracted with ethyl acetate (3x 200ml), then washed, separated and purified by silica gel column chromatography aq.NaHCO3 (6N) (EtOAc / PE = 1: 1) to give a yellow solid compound 7-2 (22.2g, 85%).
References:
Ningbo Wenda Pharmaceutical Technology Co., Ltd;Wang, Nenghui CN106146482, 2016, A Location in patent:Paragraph 0115; 0116; 0117

40925-68-6
263 suppliers
$6.00/1g

79-04-9
384 suppliers
$12.00/5g

24036-52-0
190 suppliers
$9.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24036-52-0
190 suppliers
$9.00/250mg
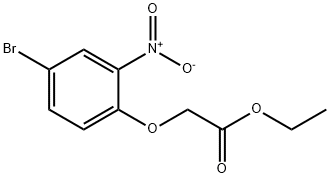
528892-33-3
11 suppliers
inquiry

24036-52-0
190 suppliers
$9.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24036-52-0
190 suppliers
$9.00/250mg