
2-(4-NITRO-PHENYL)-IMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE synthesis
- Product Name:2-(4-NITRO-PHENYL)-IMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE
- CAS Number:817172-44-4
- Molecular formula:C14H9N3O3
- Molecular Weight:267.24

504-29-0
562 suppliers
$5.00/5G

1734-79-8
106 suppliers
$9.00/250mg
![2-(4-NITRO-PHENYL)-IMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/817172-44-4.gif)
817172-44-4
35 suppliers
inquiry
Yield:817172-44-4 51%
Reaction Conditions:
with N-iodo-succinimide in water monomer at 80; for 6 h;Green chemistry;
Steps:
4.3 Typical procedure for the synthesis of 2-arylimidazo [1,2-a]pyridines
General procedure: A mixture of 2-aminopyridine 1a (94.11mg, 1mmol) and acetophenone 2a (120.15mg, 1mmol, 1 equiv.) in aqueous medium was stirred and heated at 80°C for 6h, after addition of NIS (224.98mg, 1mmol, 1 equiv.). After completion of the reaction (TLC), the mixture was cooled to room temperature and diluted with Et2O (10mL) and transferred into a separatory funnel. The organic layer was collected and further extracted with Et2O (2×10mL). The combined organic extract were dried (anh Na2SO4), filtered, then filtrate concentrated under rotary vacuum evaporation, and the residue was charged on to chromatography (100-200 mesh silica gel) column and eluted with EtOAc-hexane to afford pure 3a (192.36mg, 90%). All the remaining reactions were performed following this general procedure.
References:
Jahan, Kousar;Sofi, Firdoos Ahmad;Salim, Sumi Aisha;Bharatam, Prasad V. [Tetrahedron,2022,vol. 112,art. no. 132715]
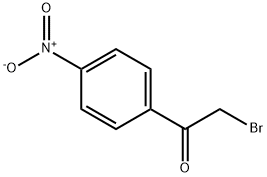
99-81-0
222 suppliers
$9.00/1g
![2-(4-NITRO-PHENYL)-IMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/817172-44-4.gif)
817172-44-4
35 suppliers
inquiry

100-19-6
309 suppliers
$5.00/5g
![2-(4-NITRO-PHENYL)-IMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/817172-44-4.gif)
817172-44-4
35 suppliers
inquiry

504-29-0
562 suppliers
$5.00/5G
![2-(4-NITRO-PHENYL)-IMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/817172-44-4.gif)
817172-44-4
35 suppliers
inquiry