
4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE synthesis
- Product Name:4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE
- CAS Number:885618-33-7
- Molecular formula:C13H17BN2O2
- Molecular Weight:244.1
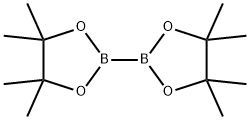
73183-34-3

186407-74-9
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
To a solution of 4-bromo-1H-indazole (500 mg, 2.54 mmol) and bis(pinacolato)diboron (1.5 eq, 3.81 mmol) in dimethyl sulfoxide (DMSO, 20 mL) was added dry potassium acetate (3.0 eq, 7.61 mmol, 747 mg) and PdCl2(dppf)2 (3 mol%, 0.076 mmol, 62 mg). The reaction mixture was degassed with argon and the reaction was heated at 80 °C for 40 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was partitioned between water (50 mL) and ether (3 x 50 mL). The organic layers were combined, washed with brine (50 mL), separated and dried over anhydrous magnesium sulfate (MgSO4). The crude product was purified by column chromatography using 30%-40% ethyl acetate/petroleum ether as eluent to give a non-separable 3:1 mixture of 1H-indazole-4-boronic acid pinacol ester (369 mg, 60%) and unreacted 4-bromo-1H-indazole (60 mg, 20%). The mixture was a yellow gel that solidified to an off-white solid upon standing.1H NMR (400 MHz, DMSO-d6) data were as follows:1H-indazole-4-boronic acid pinacol ester (70): δ 1.41 (12H, s), 7.40 (1H, dd, J = 8.4 Hz, 6.9 Hz), 7.59 (1H, d, J = 8.4 Hz), 7.67 (1H, d, J = 6.9 Hz), 10.00 (1H, br s), 8.45 (1H, s); 4-bromo-1H-indazole: δ 7.40 (1H, m), 7.18 (1H, t, J = 7.9 Hz), 7.50 (1H, d, J = 9.1 Hz), 7.77 (1H, d, J = 7.9 Hz), 8.09 (1H, s). The impurity peak appeared at δ 1.25.

73183-34-3
587 suppliers
$6.00/5g

186407-74-9
320 suppliers
$5.00/1g
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
142 suppliers
$16.00/100mg
Yield: 60%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in dimethyl sulfoxide at 80; for 40 h;Product distribution / selectivity;
Steps:
5
To a solution of the 4-bromoindazole (500 mg, 2.54mmol) and bis(pinacolato)diboron (1.5 eq., 3.81mmol) in DMSO (2OmL) was added potassium acetate (3.0 eq., 7.61mmol, 747 mg; dried in drying pistol) and PdCl2(dppf)2 (3 mol%, 0.076mmol, 62 mg). The mixture was degassed with argon and heated at 800C for 40 h. The reaction mixture was allowed to cool and partitioned between water (5OmL) and ether (3 x 5OmL). The combined organic layers were washed with brine (5OmL), separated and dried (MgSO4). The crude material was purified by chromatography eluting with 30%-»40% EtO Ac-petrol to give an inseparable 3:1 mixture of the boronate ester (369 mg, 60%) and indazole (60 mg, 20%); this was isolated as a yellow gum which solidified upon standing to furnish (70) as an off- white solid. 1H NMR (400 MHz, J6-DMSO) (70) 1.41 (12H5 s), 7.40 (IH, dd5 J=8.4Hz, 6.9Hz)5 7.59 (IH, d, J=8.4Hz), 7.67 (IH5 d, J=6.9Hz), 10.00 (IH5 br s), 8.45 (IH5 s), and indazole: 7.40 (IH5 1), 7.18 (IH5 t, J=7.9Hz)5 7.50 (IH5 d5 J=9. IHz)5 7.77 (IH5 d5 J=7.9Hz)5 8.09 (IH5 s). Impurity at 1.25.
References:
LUDWIG INSTITUTE FOR CANCER RESEARCH;CANCER RESEARCH TECHNOLOGY LIMITED;INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL;ASTELLAS PHARMA INC;PIRAMED LIMITED WO2007/42806, 2007, A1 Location in patent:Page/Page column 34-35

73183-34-3
587 suppliers
$6.00/5g

186407-74-9
320 suppliers
$5.00/1g

271-44-3
268 suppliers
$6.00/250mg
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
142 suppliers
$16.00/100mg

41748-71-4
144 suppliers
$19.00/250mg

73183-34-3
587 suppliers
$6.00/5g
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
142 suppliers
$16.00/100mg
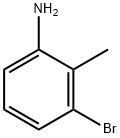
55289-36-6
294 suppliers
$5.00/1g
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
142 suppliers
$16.00/100mg

885698-70-4
37 suppliers
$115.00/500mg
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
142 suppliers
$16.00/100mg