
ALPHA-HYDROXYHIPPURIC ACID synthesis
- Product Name:ALPHA-HYDROXYHIPPURIC ACID
- CAS Number:16555-77-4
- Molecular formula:C9H9NO4
- Molecular Weight:195.17

55-21-0
409 suppliers
$5.00/5G
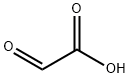
298-12-4
468 suppliers
$5.00/25g

16555-77-4
58 suppliers
$14.14/500mgs:
Yield:16555-77-4 100%
Reaction Conditions:
in water;acetone; for 19 h;Heating / reflux;
Steps:
7.7.3
The titled compound 65 was prepared according to a procedure described by Zoller et al.209 A mixture of benzamide 35 (5.00 g, 41.3 mmol) and glyoxylic acid monohydrate 41 (4.32 g, 46.9 mmol) in anhydrous acetone (70 mL) was heated at reflux for 19 h. The reaction mixture was evaporated under reduced pressure to afford the desired product 65 as a colourless solid (8.06 g, 100%), m.p. 198-200° C. (lit.209 200-201° C. (dec)) Spectroscopic data indicated the crude product 65 did not require purification and was used directly in the subsequent reaction (Section 7.11.4). νmax (KBr): 3310bs, 3058w, 1728s, 1646s, 1602w, 1582w, 1535s, 1491w, 1452w, 1315m, 1292w, 1254m, 1161m, 1097s, 1040m, 1002w, 957m, 805w, 770w, 728m, 692m, 654m, 609w, 515m, 483w cm-1. 1H n.m.r. (300 MHz, D6-DMSO): δ 5.60 (d, J=7.8 Hz, 1H, H2), 7.41-7.49 (m, 2H, H3', 5'), 7.55 (m, 1H, H4'), 7.86-7.92 (m, 2H, H2', 6'), 9.26 (d, J=7.8 Hz, 1H, NH), two exchangeable OH protons not observed. 13C n.m.r. (75 MHz, D6-DMSO): δ 71.7 (C2), 127.6, 128.3, 131.7 (Arom CH), 133.7 (C1'), 166.0, 171.5 (C1, CONH). Mass Spectrum (ESI+, MeOH): m/z 218.2 (M+Na)+, C9H9NNaO4 requires 218.2.
References:
US2007/197429,2007,A1 Location in patent:Page/Page column 58
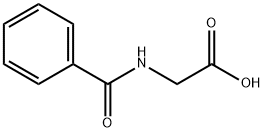
495-69-2
449 suppliers
$7.00/25g

16555-77-4
58 suppliers
$14.14/500mgs:

1199-01-5
95 suppliers
$28.60/25mg

16555-77-4
58 suppliers
$14.14/500mgs:

55-21-0
409 suppliers
$5.00/5G

563-96-2
304 suppliers
$6.00/25g

16555-77-4
58 suppliers
$14.14/500mgs:

1199-01-5
95 suppliers
$28.60/25mg
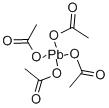
546-67-8
239 suppliers
$10.00/10g
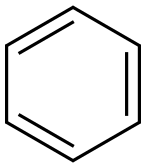
71-43-2
719 suppliers
$11.20/25ML

16555-77-4
58 suppliers
$14.14/500mgs: