
D-alpha-Amino-epsilon-caprolactam synthesis
- Product Name:D-alpha-Amino-epsilon-caprolactam
- CAS Number:28957-33-7
- Molecular formula:C6H12N2O
- Molecular Weight:128.17
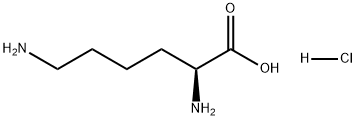
657-27-2

28957-33-7
General procedure for the synthesis of (R)-3-amino-2-caprolactam from L-lysine hydrochloride: 30 g (164 mmol) of L-lysine hydrochloride and 150 mL of 1,2-propanediol as a solvent were added to a 250 mL three-neck flask. Under stirring conditions, 6.57 g of sodium hydroxide was slowly added with continuous stirring until complete dissolution. Subsequently, the reaction mixture was heated to reflux and the reflux reaction was maintained for 5 hours, during which time the water generated was removed by means of a water separator and the progress of the reaction was monitored by thin-layer chromatography (TLC) until the reaction was complete. Upon completion of the reaction, the mixture was cooled to room temperature and the by-product sodium chloride was removed by filtration. The filtrate was acidified dropwise by adding 6 mol/L aqueous hydrochloric acid to the filtrate, followed by a back-extraction operation. The aqueous phase was collected, dried over anhydrous sodium sulfate, and recrystallized by adding appropriate amount of ethanol to finally obtain 21.9 g of white solid product (R)-α-amino caprolactam in 81% yield.

7274-88-6
411 suppliers
$14.00/5G

28957-33-7
141 suppliers
$45.00/50mg
Yield: 15%
Reaction Conditions:
with hexamethyldisilazan in toluene for 168 h;Heating / reflux;
Steps:
2.a
a) 3R-Amino-azepan-2-one D-Lysine hydrochloride (48 g, 0.26 mol) and hexamethyldisilazane (424.4 g, 2.6 mol) were suspended in toluene (1.1 litre) and refluxed for 1 week under an argon atmosphere, using a water-trap condenser. The reaction mixture was cooled to r.t. and slowly added to ice-cold MeOH (2.24 litre). The resultant clear solution was stirred for 30 min and concentrated under reduced pressure. The white, waxy solid was stirred in ethyl acetate, filtered and the filtrated concentrated under reduced pressure: white solid 5.4 g, (15%); 1H NMR (CDCl3) δ 1.35-2.05 (m, 8H), 3.2-3.26 (m, 2H), 3.53 (dd, 1H), 6.10 (br, 1H) MS: m/e=129.2 (MH+)
References:
Galley, Guido;Kitas, Eric Argirios;Jakob-Roetne, Roland US2006/14945, 2006, A1 Location in patent:Page/Page column 41

108491-55-0
4 suppliers
inquiry

28957-33-7
141 suppliers
$45.00/50mg
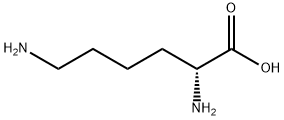
923-27-3
233 suppliers
$9.00/250mg

28957-33-7
141 suppliers
$45.00/50mg

17929-90-7
85 suppliers
inquiry

28957-33-7
141 suppliers
$45.00/50mg

21568-87-6
142 suppliers
$45.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

28957-33-7
141 suppliers
$45.00/50mg