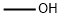Methyl 2-bromobenzoate synthesis
- Product Name:Methyl 2-bromobenzoate
- CAS Number:610-94-6
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04

88-65-3
365 suppliers
$5.00/5g

119-36-8
1051 suppliers
$5.00/10g

610-94-6
314 suppliers
$13.00/25g
69-72-7
1315 suppliers
$5.00/10g
Yield:610-94-6 91%
Reaction Conditions:
Stage #1:2-bromobenzoic-acid with potassium carbonate in N,N-dimethyl acetamide at 110; for 0.5 h;
Stage #2:methyl salicylate at 110; for 24 h;
Steps:
Methyl 2-Methoxybenzoate (3a); Typical Procedure
General procedure: A mixture of 2-methoxybenzoic acid (3.8 g, 25 mmol) and K2CO3 (2.07 g, 15 mmol) in DMA (50 mL) was stirred at 110 °C for 0.5 h. Methyl salicylate (5.70 g, 37.5 mmol) was added and the resulting mixture was stirred for 24 h. The solvent was then removed in vacuo. After cooling to r.t., K2CO3 (2.42 g, 17.5 mmol) and water (50mL) were added to hydrolyze the excess methyl salicylate. The resulting mixture was heated at 60 °C until methyl salicylate disappeared on TLC. Then, the solution was extracted with EtOAc (3 ×20 mL). The organic layer was washed with water, sat. aq NaCl solution,and dried (anhyd MgSO4). Evaporation of solvent in vacuoafforded methyl 2-methoxybenzoate (3.82 g, 92%). More than 90% of salicylic acid was recovered as a white precipitate by acidifying the aqueous phase with 1 M HCl.
References:
Chen, Si;Jia, Lei;Li, Xiaonan;Luo, Meiming [Synthesis,2014,vol. 46,# 2,art. no. SS-2013-H0569-OP,p. 263 - 268]

75-91-2
215 suppliers
$26.00/100g

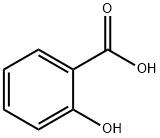
95-46-5
301 suppliers
$10.00/10 g

610-94-6
314 suppliers
$13.00/25g

93-58-3
508 suppliers
$13.50/100 mL

610-94-6
314 suppliers
$13.00/25g

75-91-2
215 suppliers
$26.00/100g

6630-33-7
425 suppliers
$9.00/10g

610-94-6
314 suppliers
$13.00/25g