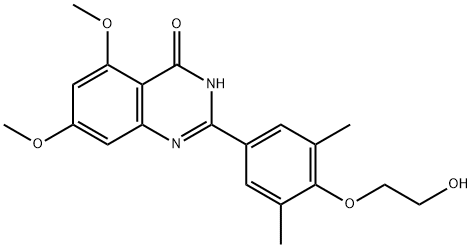
RVX-208 synthesis
- Product Name:RVX-208
- CAS Number:1044870-39-4
- Molecular formula:C20H22N2O5
- Molecular Weight:370.4

1039948-89-4
59 suppliers
$45.00/50mg

63920-73-0
64 suppliers
$50.00/50 mg

1044870-39-4
163 suppliers
$35.00/1mg
Yield: 79.9%
Reaction Conditions:
with toluene-4-sulfonic acid;sodium hydrogensulfite in ISOPROPYLAMIDE at 115;
Steps:
1
Intermediate 2 (58.74 kg), ?/,?/-dimethylacetamide (280 kg), and starting material 3 (56.00 kg) were combined and p-toluenesulfonic acid monohydrate (5.90 kg) and 1/3 of the required sodium bisulfite (24.1 kg) were added. The mixture was heated to 115 0C and stirred for 90-105 minutes before the second 1/3 of the required sodium bisulfite (24.1 kg) was added. The remaining sodium bisulfite (24.1 kg) was added after another 90-105 minutes. The reaction mixture was stirred at 115 0C until the reaction was complete as determined by HPLC (approximately 1 hour, less than 4% of intermediate 2 remaining). The reaction mixture was cooled to 25 0C and added to water (1770 kg). The mixture was stirred at 20 0C for 6 hours to complete the crystallization. The crude material was isolated by filtration, washed with water (234 kg) and dried under vacuum to constant weight. The crude material was dissolved in ?/,?/-dimethylacetamide (252 kg) at 80 0C until all material had dissolved. The solution was cooled to 60 0C and heptane (918 kg) was slowly added over a period of 1 hour, maintaining a temperature above 35 0C.The solution was cooled to 35 0C and stirred at 35 0C for a minimum of 1 hour. The solid was isolated by filtration, washed with heptane (250 kg) and dried to constantweight under vacuum. Yield: 92.5%; purity: 98.6%. The dry solid (83.1 kg) was added to a 1 :1 mixture of ethanol and water (1V/1V; 1670 kg), and the mixture was heated to approximately 840C (reflux) until all material was in solution. The solution was cooled to 70 0C and polish-filtered, and then cooled to 30 0C over 2 hours. The solution was cooled to 0 0C. The mixture was stirred at 0 0C for at least 1 hour, before the material was isolated by filtration, washed with ethanol/water (1V/1V; 33 kg) and dried under vacuum to constant weight. The material was passed through a 60-mesh screen to afford 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7- dimethoxyquinazolin-4(3H)-one (4). Yield: 66.4 kg; 79.9%.
References:
RESVERLOGIX CORP. WO2009/158404, 2009, A1 Location in patent:Page/Page column 16-17

96-49-1
519 suppliers
$6.00/100g
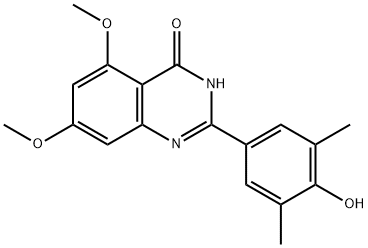
1044870-30-5
2 suppliers
inquiry

1044870-39-4
163 suppliers
$35.00/1mg

1044872-73-2
7 suppliers
inquiry

62827-46-7
1 suppliers
inquiry

1044870-39-4
163 suppliers
$35.00/1mg

1039948-89-4
59 suppliers
$45.00/50mg

1545025-44-2
3 suppliers
inquiry

1044870-39-4
163 suppliers
$35.00/1mg

1044872-73-2
7 suppliers
inquiry

63920-73-0
64 suppliers
$50.00/50 mg

1044870-39-4
163 suppliers
$35.00/1mg