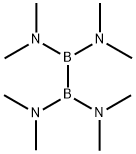
TETRAHYDROXYDIBORON synthesis
- Product Name:TETRAHYDROXYDIBORON
- CAS Number:13675-18-8
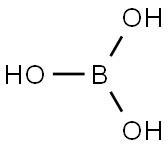
- Molecular formula:B2H4O4
- Molecular Weight:89.65

7732-18-5

158752-98-8

13675-18-8
Tetrakis(pyrrolidino)diborane (0.1 mole) was added to a three-necked flask equipped with a titration and refluxing device under a nitrogen atmosphere with 50-60 times the volume of electrophoretic grade deionized water. The temperature of the reaction system was raised to 100 °C and the reaction was refluxed for 1.5 hours. Subsequently, the unit was replaced with an atmospheric pressure distillation unit and distilled to remove the tetrahydropyrrole generated in the reaction. After the tetrandrine was completely evaporated, the reaction solution was cooled to room temperature, at which point a solid precipitated. The solid was collected by filtration and dried to give 8.5 g of white solid tetrahydroxydiboron, which was analyzed by HNMR to confirm that the purity was greater than 98%, which was consistent with literature reports. The resulting tetrahydroxydiboron was dissolved in 45 mL of methanol and stirred at room temperature until completely dissolved (took about 10-15 min). Subsequently, aqueous KHF2 solution (0.38 mol) was added and stirring was continued for 1 h at room temperature. After completion of the reaction, the system was left to stratify and the lower liquid was carefully separated. The lower liquid was distilled to dryness, acetone was added and the mixture was filtered to give 15.8 g of white solid potassium boron trifluoride in 74% overall yield.

7732-18-5
507 suppliers
$12.70/100ml

158752-98-8
8 suppliers
inquiry

13675-18-8
166 suppliers
$6.00/1g
Yield:13675-18-8 8.5 g
Reaction Conditions:
at 100;Inert atmosphere;
Steps:
2 Example 2
Under nitrogen protection, tetrakis (tetrahydropyrrole) boron (0.1) was added to a three-necked flask equipped with a dropping and refluxing unitMole) and 50-60 times deionized water, and the temperature was raised to 100 ° C. After refluxing for 1.5 hours, the mixture was replaced by an atmospheric distillation unit,The reaction process to generate tetrahydropyrrole distillation, no longer with tetrahydropyrrole distilled, the reaction solution is cooled to room temperature, at this time a solid precipitation,Filtered and dried to give 8.5 g of whitesolidTetrahydroxy boron, HNMR purity of more than 98%, consistent with the literature.Add 45 ml of methanol, stir at room temperature until completely dissolved (about 10-15 minutes), then add KHF2 aqueous solution (0.38 mol), stirring at room temperature for 1 hour, the system after standing and layered, carefully separated from the lower, Distilled to dry. After addition of acetone, the mixture was filtered to give 15.8 g of potassium boron trifluoride as a white solid, the total yield was 74%
References:
Cangzhou Purui Dongfang Science and Technology Co., Ltd.;Li, Juanjuan;Guo, Shixue;Leng, Yanguo CN104945426, 2017, B Location in patent:Paragraph 0026; 0027; 0028
13826-27-2
141 suppliers
$5.00/250mg

13675-18-8
166 suppliers
$6.00/1g

120-80-9
532 suppliers
$13.00/25g

13061-96-6
304 suppliers
$6.00/1g
73183-34-3
587 suppliers
$6.00/5g

13675-18-8
166 suppliers
$6.00/1g

7732-18-5
507 suppliers
$12.70/100ml
13701-67-2
3 suppliers
inquiry

13675-18-8
166 suppliers
$6.00/1g
11113-50-1
297 suppliers
$34.41/100 g