| Identification | Back Directory | [Name]
1,1'-BIS(DI-TERT-BUTYLPHOSPHINO)FERROCENE | [CAS]
84680-95-5 | [Synonyms]
DBtPF
98% DtBPF
1,1'-Bis(di-tert-butylphosphin
1,1'-BIS(DI-T-BUTYLPHOSPHINO)FERROCENE
1,1'-BIS(DI-TERT-BUTYLPHOSPHINO)FERROCENE
1,1'-Bis(di-tert-butylphosphiNA)ferrocene
1,1'-Bis(di-t-butylphosphino)ferrocene,DtBPF
1,1'-Bis(di-tert-butylphosphino)ferrocene ,98%
1,1'-Bis(di-t-butylphosphino)ferrocene,min.98%
1,1'-Bis(di-t-butylphosphino)ferrocene, min. 98%
BIS[(DI-TERT-BUTYLPHOSPHINO)CYCLOPENTADIENYL]IRON
1,1'-Bis(di-t-butylphosphino)ferrocene,98% DtBPF
1,1'-Bis(di-tert-butylphosphino)ferrocene,min. 98%
1,1'-Bis[bis(1,1-diMethylethyl)phosphino]ferrocene
1,1'-Bis(di-t-butylphosphino)ferrocene, min. 98% DTBPF | [EINECS(EC#)]
1533716-785-6 | [Molecular Formula]
C26H44FeP2 10* | [MDL Number]
MFCD01630818 | [MOL File]
84680-95-5.mol | [Molecular Weight]
474.42 |
| Chemical Properties | Back Directory | [Melting point ]
181-182°C (dec.) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
crystal | [color ]
orange to red | [Water Solubility ]
Insoluble in water. | [InChI]
InChI=1S/2C13H22P.Fe/c2*1-12(2,3)14(13(4,5)6)11-9-7-8-10-11;/h2*7-10H,1-6H3; | [InChIKey]
FPLSJBJGQLJLSV-UHFFFAOYSA-N | [SMILES]
P(C(C)(C)C)(C(C)(C)C)[C]1[CH][CH][CH][CH]1.P(C(C)(C)C)(C(C)(C)C)[C]1[CH][CH][CH][CH]1.[Fe] |^1:9,10,11,12,13,23,24,25,26,27| |
| Hazard Information | Back Directory | [Chemical Properties]
1,1'-Bis(di-tert-butylphosphino)ferrocene has good stability and reactivity and can participate in a variety of organometallic catalytic reactions, such as diene complex formation, carbonyl insertion, addition, reduction and deoxygenation reactions.
| [Uses]
1,1'-BIS(DI-TERT-BUTYLPHOSPHINO)FERROCENE is an organophosphine compound and can be used as an organometallic ligand. | [Uses]
The rate of palladium-catalyzed amination of unactivated aryl chlorides is accelerated by sterically hindered chelating alkyl phosphines, ie, 1,1'-bis(di-tert-butylphosphino)ferrocene. | [reaction suitability]
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Indole Forming Reactions
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling |
| Questions And Answer | Back Directory | [Reaction]
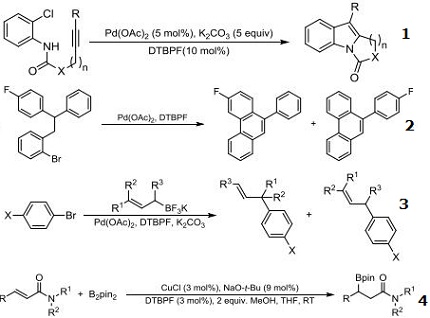
- Ligand for synthesis of polycyclic indoles via Pd-catalyzed intramolecular heteroannulation.
- Ligand for the palladium-catalyzed intramolecular arylation of aryl bromides under mild conditions.
- Ligand for cross-coupling reactions between bromoarenes and potassium allyltrifluoroborates promoted by a catalyst prepared from Pd(OAc)2 and DTBPF selectively providing γ-coupling products.
- Ligand for the copper-catalyzed system for the ß-boration of of a variety of α,ß-unsaturated amides.
- Ligand for the synthesis of Paucifloral F and related indanone analogues via palladium-catalyzed α-arylation.
- Ligand for the Pd-carbon monoxide complex catalyzed hydroxycarbonylation of aryl halides.
- Ligand for the palladium-catalyzed β-C-glycosylation by decarboxylative allylation to normal pyran systems,and cis-2,6-disubstituted tetrahydropyrans.
- Pd-catalyzed dearomative indole bisfunctionalization via a diastereoselective arylcyanation.
- Ligand for the copper- DTBPF catalyzed C–H activation and carboxylation of terminal alkynes.

|
|
|