130753-13-8
 130753-13-8 结构式
130753-13-8 结构式
基本信息
N-CBZ-降托品酮
N-CBZ-去甲托品酮
N-苄氧羰基去甲托品酮
N-苄氧羰基-去甲拖品酮
3-氧代-8-氮杂双环[3,2,1]辛-8-羧酸苄酯
3-氧代-8-氮杂双环[3,2,1]辛烷-8-羧酸苄酯
N-苄氧羰基-去甲托品酮(CAS号:130753-13-8)
N-Cbz-Notropinone
N-CBZ-NORTROPINONE
N-Cbz-4-Nortropinone
N-Cbz-nortropinone ,97%
8-Cbz-3-oxo-8-azabicyclo[3.2.1]octane
Benzyl 3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate
benzyl (1s)-3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate
(1S)-BENZYL 3-OXO-8-AZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE
Benzyl (1S,5R)-3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate
物理化学性质
制备方法
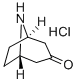
25602-68-0
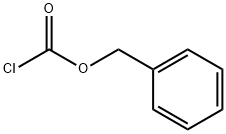
501-53-1

130753-13-8
1. 将8-氮杂双环[3,2,1]辛-3-酮盐酸盐(10.0g; 71.84mmol)溶于二氯乙烷(DCE, 60mL)中,缓慢滴加氯甲酸1-氯乙酯(ACE-C1, 14.5mL; 19.11g; 133.7mmol)。反应混合物在室温下搅拌过夜后,用乙醚(Et2O, 400mL)稀释并过滤。滤液经减压浓缩,得到粗制的氯乙基氨基甲酸酯。 2. 将上述粗产物溶于甲醇(MeOH, 200mL)中,室温搅拌1小时,随后在55℃下减压浓缩,得到粗制的去甲基托品酮盐酸盐(褐色固体,11.4g,收率98%)。 3. 粗产物通过甲腈重结晶纯化,得到白色结晶固体(5g,收率43%)。1H NMR(400MHz, DMSO-d6)δ1.79(dd, J = 15.0,6.9Hz, 2H), 2.09(m, 2H), 2.40(d, J = 16.7Hz, 2H), 3.02(dd, J = 17.1,4.3Hz, 2H), 4.23(s, 2H), 10.00(br s, 2H)。 4. 将去甲基托品酮(5.10g; 31.55mmol)溶于二氯甲烷(CH2Cl2, 50mL)中,缓慢滴加氯甲酸苄酯(4.29mL; 5.11g; 29.98mmol)和二异丙基乙胺(DIPEA, 16.48mL; 12.23g; 94.66mmol)(注意放热反应)。反应混合物在室温下搅拌30分钟后,用二氯甲烷(100mL)稀释。 5. 有机相用1N盐酸(2×100mL)洗涤,无水硫酸钠(Na2SO4)干燥后减压浓缩,得到粗产物(7.2g,收率88%)。1H NMR(400MHz, CDCl3)δ1.71(dd, J = 15.0,7.2Hz, 2H), 2.12(m, 2H), 2.38(d, J = 15.9Hz, 2H), 2.67(m, 2H), 4.62(s, 2H), 5.22(s, 2H), 7.38(m, 5H)。
参考文献:
[1] Patent: WO2006/23852, 2006, A2. Location in patent: Page/Page column 84
[2] Patent: JP2015/124178, 2015, A. Location in patent: Paragraph 0143
[3] Patent: WO2004/100946, 2004, A1. Location in patent: Page 77
[4] Patent: WO2006/58303, 2006, A2. Location in patent: Page/Page column 71
[5] Patent: WO2010/126163, 2010, A1. Location in patent: Page/Page column 38-39
