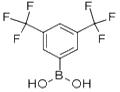
3,5-Bis(trifluoromethyl)benzeneboronic acid
- CAS No.
- 73852-19-4
- Chemical Name:
- 3,5-Bis(trifluoromethyl)benzeneboronic acid
- Synonyms
- 3,5-BIS(TRIFLUOROMETHYL)PHENYLBORONIC ACID;AKOS BRN-0072;RARECHEM AH PB 0029;3,5-BIS(TRIFLUOROMETHYL)PHENYLBORONIC;3,5-Bis(trifluoroMethyl)benzeneboronic;5-Bis(trifluoroMethyl)phenylboronic acid;3,5-Bis(trifloroMethyl)phenylboronic acid;3,5-DI(TRIFLUOROMETHYL)PHENYLBORONIC ACID;3,5--TrifluoroMethyl benzene boronic acid;3,5-BIS(TRIFLUOROMETHYL)PHENYLBOROIC ACID
- CBNumber:
- CB6205984
- Molecular Formula:
- C8H5BF6O2
- Molecular Weight:
- 257.93
- MDL Number:
- MFCD00051850
- MOL File:
- 73852-19-4.mol
| Product description | Number | Pack Size | Price |
| 3,5-Bis(trifluoromethyl)phenylboronic acid ≥95% | 471070 | 5g | $78.27 |
| 3,5-Bis(trifluoromethyl)phenylboronic Acid (contains varying amounts of Anhydride) | B1886 | 1g | $21 |
| 3,5-Bis(trifluoromethyl)phenylboronic Acid (contains varying amounts of Anhydride) | B1886 | 5g | $58 |
| 3,5-Bis(trifluoromethyl)phenylboronic Acid (contains varying amounts of Anhydride) | B1886 | 25g | $172 |
| 3,5-Bis(trifluoromethyl)phenylboronic acid | B412868 | 5g | $55 |
| More product size | |||
| Melting point | 217-220 °C (lit.) |
|---|---|
| Boiling point | 248.1±50.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | soluble in Methanol |
| pka | 6.57±0.10(Predicted) |
| form | Crystals or Powder |
| color | White to light yellow |
| BRN | 7379990 |
| InChI | InChI=1S/C8H5BF6O2/c10-7(11,12)4-1-5(8(13,14)15)3-6(2-4)9(16)17/h1-3,16-17H |
| InChIKey | BPTABBGLHGBJQR-UHFFFAOYSA-N |
| SMILES | B(C1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1)(O)O |
| CAS DataBase Reference | 73852-19-4(CAS DataBase Reference) |
| UNSPSC Code | 12352103 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a-P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313 | |||||||||
| PPE | Eyeshields, Gloves, type N95 (US) | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 37/39-26 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | No | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29163990 | |||||||||
| Storage Class | 11 - Combustible Solids | |||||||||
| NFPA 704 |
|
3,5-Bis(trifluoromethyl)benzeneboronic acid price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 471070 | 3,5-Bis(trifluoromethyl)phenylboronic acid ≥95% | 73852-19-4 | 5g | $78.27 | 2025-07-31 | Buy |
| TCI Chemical | B1886 | 3,5-Bis(trifluoromethyl)phenylboronic Acid (contains varying amounts of Anhydride) | 73852-19-4 | 1g | $21 | 2025-07-31 | Buy |
| TCI Chemical | B1886 | 3,5-Bis(trifluoromethyl)phenylboronic Acid (contains varying amounts of Anhydride) | 73852-19-4 | 5g | $58 | 2025-07-31 | Buy |
| TCI Chemical | B1886 | 3,5-Bis(trifluoromethyl)phenylboronic Acid (contains varying amounts of Anhydride) | 73852-19-4 | 25g | $172 | 2025-07-31 | Buy |
| TRC | B412868 | 3,5-Bis(trifluoromethyl)phenylboronic acid | 73852-19-4 | 5g | $55 | 2021-12-16 | Buy |
3,5-Bis(trifluoromethyl)benzeneboronic acid Chemical Properties,Uses,Production
Chemical Properties
Off-white Cryst
Uses
suzuki reaction
Uses
Reactant involved in the synthesis of:
- Methylene-arylbutenones via carbonylative arylation of allenols
- 4-aminoquinoline analogs via Ullman / Suzuki / Negishi coupling
- Primary amino acid derivatives with anticonvulsant activity
- Alkyl arylcarbamates via Cu-catalyzed coupling with potassium cyanate
- Aryl-substituted succinimides and cyclic ketones by asymmetric conjugate addition
- Axially chiral dicarboxylic acids for asymmetric Mannich-type reactions
Synthesis

121-43-7
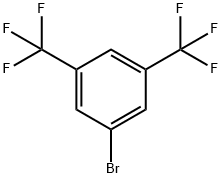
328-70-1
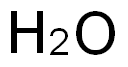
7732-18-5

73852-19-4
General procedure for the synthesis of 3,5-bis(trifluoromethyl)benzeneboronic acid from trimethyl borate, 3,5-bis(trifluoromethyl)bromobenzene, and electrophoretic-grade water: in a three-necked, dry, round-bottomed flask equipped with a rod-shaped magnetic stirrer, a 500-ml dropping funnel, condenser tube, and nitrogen introduction tube, 9.30 g of magnesium flakes were added under nitrogen protection and 220 ml of tetrahydrofuran (THF), which had been distillate-purified, were added ). The temperature of the reaction system was maintained in the range of 0 to 5°C by means of an ice bath. At this temperature, 44.0 mL of 3,5-bis(trifluoromethyl)bromobenzene was slowly added through a dropping funnel and the addition process was controlled to be completed within 1 hour. After the addition was completed, the reaction was slowly warmed to room temperature and continued for 16 hours to obtain a dark brown viscous solution. Subsequently, the reaction solution was cooled to -70°C in a dry ice/acetone bath and 28.97 mL of trimethyl borate was slowly added dropwise through a dropping funnel. After completion of the dropwise addition, the reaction solution was allowed to warm naturally to room temperature and the reaction was continued for 12 hours to obtain a brown viscous solution. Under cooling in an ice bath, 144.5 ml of 37 wt% aqueous hydrochloric acid solution was mixed with 63.3 ml of distilled water and slowly added to the reaction solution over a period of 1 hour, after which the reaction was continued for 24 hours. Upon completion of the reaction, the red organic layer was separated using a partition funnel. The organic layer was concentrated under reduced pressure to remove the solvent and the solid residue obtained was purified by recrystallization in distilled water. The resulting white crystals were collected, washed with petroleum ether and dried at room temperature for 24 hours to afford the target product 3,5-bis(trifluoromethyl)benzeneboronic acid (6FBB) (19.44 g, yield: 85%).
References
[1] Patent: KR2015/112649, 2015, A. Location in patent: Paragraph 0089; 0093-0095
3,5-Bis(trifluoromethyl)benzeneboronic acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615350571055 | Sibel@chuanghaibio.com | China | 8753 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718; +8613336195806 | sales@capot.com | China | 29735 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-2158073036 | info@dakenam.com | China | 13636 | 58 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29833 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| Wuhan Chemwish Technology Co., Ltd | 027-67849912 | sales@chemwish.com | CHINA | 10821 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49977 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5600 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 | marketing1@neostarunited.com | China | 8830 | 58 |
View Lastest Price from 3,5-Bis(trifluoromethyl)benzeneboronic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2026-01-30 | 3,5-Bis(trifluoromethyl)benzeneboronic acid
73852-19-4
|
US $1.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2019-07-06 | 3,5-Bis(trifluoromethyl)benzeneboronic acid
73852-19-4
|
US $1.00 / KG | 1KG | 97%-99% | 100kg | Career Henan Chemical Co |
-

- 3,5-Bis(trifluoromethyl)benzeneboronic acid
73852-19-4
- US $1.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- 3,5-Bis(trifluoromethyl)benzeneboronic acid
73852-19-4
- US $1.00 / KG
- 97%-99%
- Career Henan Chemical Co
73852-19-4(3,5-Bis(trifluoromethyl)benzeneboronic acid)Related Search:
1of4





