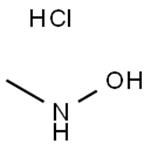
N-Methylhydroxylamine hydrochloride
- CAS No.
- 4229-44-1
- Chemical Name:
- N-Methylhydroxylamine hydrochloride
- Synonyms
- 42229-44-1;N-Methylhydroxylamin;N-METHYLHYDROXYAMINEHCL;N-METHYLHYDROXYLAMINE HCL;Methyl hydroxylamine .HCL;Methylhydroxylammonium chloride;N-Methylhydroxyaminehydrochloride;N-METHYLHYDROXYLAMMONIUM CHLORIDE;n-hydroxy-methanaminhydrochloride;N-Hydroxymethanamine hydrochloride
- CBNumber:
- CB9403590
- Molecular Formula:
- CH6ClNO
- Molecular Weight:
- 83.52
- MDL Number:
- MFCD00012597
- MOL File:
- 4229-44-1.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| N-Methylhydroxylamine hydrochloride 98% | M50400 | 5g | $65.5 |
| N-Methylhydroxylamine hydrochloride 98% | M50400 | 10g | $105 |
| N-Methylhydroxylamine Hydrochloride >97.0%(T) | M0804 | 5g | $36 |
| N-Methylhydroxylamine Hydrochloride >97.0%(T) | M0804 | 25g | $141 |
| N-Methylhydroxylamine hydrochloride | M323650 | 10g | $200 |
| More product size | |||
| Melting point | 86-88 °C(lit.) |
|---|---|
| Density | 1.36 g/cm3 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 0.1 g/mL, clear, very faintly yellow |
| form | solid |
| color | white |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| BRN | 3541409 |
| InChI | InChI=1S/CH5NO.ClH/c1-2-3;/h2-3H,1H3;1H |
| InChIKey | RGZRSLKIOCHTSI-UHFFFAOYSA-N |
| SMILES | N(O)C.Cl |
| CAS DataBase Reference | 4229-44-1(CAS DataBase Reference) |
| FDA UNII | MGW04SJ1LZ |
| EPA Substance Registry System | Methanamine, N-hydroxy-, hydrochloride (4229-44-1) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P302+P352-P305+P351+P338 | |||||||||
| target organs | Respiratory system | |||||||||
| PPE | dust mask type N95 (US), Eyeshields, Gloves | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-24/25 | |||||||||
| RIDADR | 1759 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 3-10 | |||||||||
| TSCA | TSCA listed | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29280090 | |||||||||
| Storage Class | 11 - Combustible Solids | |||||||||
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |
|||||||||
| NFPA 704 |
|
N-Methylhydroxylamine hydrochloride price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M50400 | N-Methylhydroxylamine hydrochloride 98% | 4229-44-1 | 5g | $65.5 | 2025-07-31 | Buy |
| Sigma-Aldrich | M50400 | N-Methylhydroxylamine hydrochloride 98% | 4229-44-1 | 10g | $105 | 2025-07-31 | Buy |
| TCI Chemical | M0804 | N-Methylhydroxylamine Hydrochloride >97.0%(T) | 4229-44-1 | 5g | $36 | 2025-07-31 | Buy |
| TCI Chemical | M0804 | N-Methylhydroxylamine Hydrochloride >97.0%(T) | 4229-44-1 | 25g | $141 | 2025-07-31 | Buy |
| TRC | M323650 | N-Methylhydroxylamine hydrochloride | 4229-44-1 | 10g | $200 | 2021-12-16 | Buy |
N-Methylhydroxylamine hydrochloride Chemical Properties,Uses,Production
Chemical Properties
The substance appears as white to almost white crystals or crystalline powder. It exhibits solubility in water.
Uses
N-Methylhydroxylamine hydrochloride is used as an inorganic catalyst used in the transamidation of primary amides with amines. It is employed in constructing hydroxamates, important functional groups for the complexation of iron.
Preparation
N-methylhydroxylamine hydrochloride (N-MHA) was synthesized by a high pressure catalytic hydrogenation method using noble metal Pd/C and Pt-Rh/C as the catalyst. During the synthesis process, the catalyst was easily poisoned and the products would be deeply reduced to methylamine. Therefore, the purity of N-MHA is low and the cost is high. An industrial electrolytic cell was designed for the electrochemical synthesis of N-methylhydroxylamine hydrochloride (N-MHA). Copper was used as the cathode, graphite as the anode, and a cation membrane as the separator. The results show that N-MHA with a high purity of 99% can be electrosynthesized directly from nitromethane in HCl solution.
Reactions
N-methylhydroxylamine hydrochloride was widely used in the 1,3-dipolar cycloaddition and N-methylation reaction. For example, it has been considered an extremely important intermediate in the synthesis of biological active azetidinone and isoxazole compounds such as piperylone, isopyrine, and muscimole.
Synthesis

593-77-1

4229-44-1
To a 500 mL three-necked flask equipped with a magnetic stirrer, a thermocouple and an ice water bath was added 224.0 g of crude N-methylhydroxylamine prepared in Example 1. Under the condition of keeping the reaction temperature below 25°C, 110.3 g of 37% aqueous hydrochloric acid solution (10% molar excess) was slowly added dropwise. Upon completion of the reaction, 333.4 g of a mixed methanol/water solution containing N-methylhydroxylamine hydrochloride was obtained.
References
[1] A. Gibson and S. Mirzazadeh. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs.British Journal of Pharmacology.1989, 96 637-644. DOI:10.1111/j.1476-5381.1989.tb11863.x
[2] J. R. Ochoa Gomez. Electrosynthesis of N-methylhydroxylamine.Journal of Applied Electrochemistry.1991, 21 331-334. DOI:10.1007/BF01020218
[3] Reacts with aldehydes and ketones to give nitrones, which undergo 1,3-dipolar addition reactions with alkenes. Cycloaddition to trimethylvinylsilane has been used in a 2-carbon extension of aldehydes to ɑ-unsaturated aldehydes: J. Org. Chem., 49, 3421 (1984). DOI:10.1021/jo00192a047
[4] Intramolecular cycloaddition has been used as a route to bicyclic systems, e.g. from citronellal: Org. Synth. Coll., 6, 670 (1988).
[5] Reviews: 1,3-Dipolar cycloadditions of nitrones: Synthesis, 205 (1975). The [3+2] nitrone-olefin cycloaddition reaction: Org. React., 36, 1 (1988). Synthetic applications of nitrones: Org. Prep. Proced. Int., 17, 25 (1985).
[6] For conversion to, and reactions of, the N,O-bis(TMS) derivative, see: J. Chem. Soc., Perkin 1, 1823 (1989).
[7] GAN Y, WENKUI Z, HUANG H, et al. Industrial Synthesis of N-Methylhydroxylamine Hydrochloride by Electrochemical Reduction of Nitromethane[J]. Chinese Journal of Chemical Engineering, 2006, 14: 649-653. DOI:10.1016/S1004-9541(06)60129-8.
N-Methylhydroxylamine hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81139210 +86-18192627656 | 1059@dideu.com | China | 3589 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29831 | 58 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 21193 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49977 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 +86-17392581500 | 1015@dideu.com | China | 3986 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 | marketing1@neostarunited.com | China | 8830 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 11727 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
View Lastest Price from N-Methylhydroxylamine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-12-12 | N-Methylhydroxylamine hydrochloride
4229-44-1
|
US $20.00-100.00 / KG | 1KG | 99% | 200000KG | Shaanxi Dideu Medichem Co. Ltd | |
 |
2025-04-04 | N-Methylhydroxylamine hydrochloride
4229-44-1
|
US $0.00-0.00 / KG | 1KG | 98% | 1ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2021-11-15 | N-Methylhydroxylamine hydrochloride
4229-44-1
|
US $7.00 / g | 100g | 99% | 500ton/Month | Qiuxian Baitai New Material Co., LTD |
-

- N-Methylhydroxylamine hydrochloride
4229-44-1
- US $20.00-100.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
-

- N-Methylhydroxylamine hydrochloride
4229-44-1
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- N-Methylhydroxylamine hydrochloride
4229-44-1
- US $7.00 / g
- 99%
- Qiuxian Baitai New Material Co., LTD
4229-44-1(N-Methylhydroxylamine hydrochloride)Related Search:
1of4





