- Sitafloxacin
-

- $0.00 / 1KG
-
2026-01-30
- CAS:163253-35-8
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 100kg
- Sitafloxacin
-

- $1.00 / 1KG
-
2026-01-30
- CAS:163253-35-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10mt
|
| | Sitafloxacin Sesquihydrate Basic information |
| Product Name: | Sitafloxacin Sesquihydrate | | Synonyms: | Sitafloxacin hydrate;Sitafloxacin Sesquihydrate;7-[(7S)-7-Amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-1,4-dihydro-4-oxoquinoline-3-carboxylic acid hydrate (2:3);3-Quinolinecarboxylic acid, 7-((7S)-7-amino-5-azaspiro(24)hept-5-yl)-8-chloro-6-fluoro-1-((1R,2S)-2-fluorocyclopropyl)-1,4-dihydro-4-oxo-, hydrate (2:3);3-Quinolinecarboxylic acid, 7-(7-amino-5-azaspiro(24)hept-5-yl)-8-chloro-6-fluoro-1-(2-fluorocyclopropyl)-1,4-dihydro-4-oxo-, hydrate (2:3), (1R-(1alpha(S*),2alpha))-;Du 6859a;Unii-9td681796g;Sitafloxacin hydrate (2:3) | | CAS: | 163253-35-8 | | MF: | C19H20ClF2N3O4 | | MW: | 427.83 | | EINECS: | 1308068-626-2 | | Product Categories: | DU 6859A, DU 6859, DU-6859, LS-184401;Intermediates & Fine Chemicals;Pharmaceuticals;Inhibitors | | Mol File: | 163253-35-8.mol |  |
| | Sitafloxacin Sesquihydrate Chemical Properties |
| Melting point | 145-147°C (dec.) | | alpha | 589 -199.9° | | storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C | | solubility | Aqueous Base (Slightly), DMSO (Slightly), Methanol (Very Slightly, Heated) | | form | Solid | | color | White to Off-White | | Stability: | Hygroscopic | | InChIKey | ANCJYRJLOUSQBW-UWQPBFEBNA-N | | SMILES | N[C@@H]1CN(C2=C(C=C3C(=O)C(C(=O)O)=CN([C@@H]4C[C@@H]4F)C3=C2Cl)F)CC21CC2.O |&1:1,16,18,r| | | CAS DataBase Reference | 163253-35-8(CAS DataBase Reference) |
| WGK Germany | WGK 3 | | HS Code | 2941900000 | | Storage Class | 11 - Combustible Solids |
| | Sitafloxacin Sesquihydrate Usage And Synthesis |
| Description | The fluoroquinolone antibacterial agent sitafloxacin hydrate
was developed by Daiichi Sankyo and was approved
and launched last year in Japan. Sitafloxacin’s mode of action
is through inhibition of DNA gyrase and topoisomerase
IV. It is indicated for the treatment of inflammatory infections
such as laryngopharyngitis, adenoiditis, acute bronchitis,
pneumonia, secondary infections due to chronic respiratory
lesions, cystitis, pyelonephritis, urethritis, cervicitis,
otitismedia, sinusitis, periodontitis, and pericoronitis and jaw
inflammation. Due to its broad spectrum of potent antibacterial
activity, sitafloxacin is expected to be clinically effective
in treating severe cases of bacterial infection, relapse/recrudescence of infection and infections in which resistant
bacteria are suspected to be the cause. | | Chemical Properties | Off-White Solid | | Uses | Sitafloxacin is a new-generation, broad-spectrum oral fluoroquinolone antibiotic.
It is very active against many Gram-positive, Gram-negative and anaerobic clinical isolates, including strains resistant to other fluoroquinolones, was recently approved in | | Uses | Antibacterial | | Side effects | The most commonly reported adverse reactions include rash, dizziness, headache, diarrhea, loose stool, stomach discomfort, abdominal bloating, abdominal pain, constipation, indigestion, nausea and stomatitis.
| | Synthesis | The optically pure fluorocyclopropylamine 111 intermediate
was prepared as described in thescheme. Condensation of
diphenylmethyl amine 104 with acetaldehyde followed by
treatment with trichloromethyl chloroformate in the presence
of triethylamine gave N-vinyl carbamoyl chloride 105 in
53% yield. This intermediate was reacted with sodium benzyloxide
(generated in situ) to afford N-vinylcarbamate 106
in 82% yield. Fluorocyclopropanation of 106 with zinc¨C
monofluorocarbenoid generated from fluorodiiodomethane
and diethylzinc provided N-(2-fluorocyclopropyl)carbamate
107 in 90% yield and with a diastereomeric ratio of 93:7
favoring the cis-isomer. Hydrogenolysis of the CBz and the
diphenylmethyl groups was accomplished with catalytic 10%
palladium on charcoal and was followed by treatment with
TsOH to afford dl-108 as its tosylate salt. Acylation of dl-
108 TsOH with l-menthyl chloroformate gave a 1:1 mixture
of the diastereomeric carbamate 109 which upon four repeated
recrystallizations from hexane/ethyl acetate afforded
optically pure 110 in 26% yield. Acidic hydrolysis of 110
furnished 111 as its HCl salt in 88% yield. 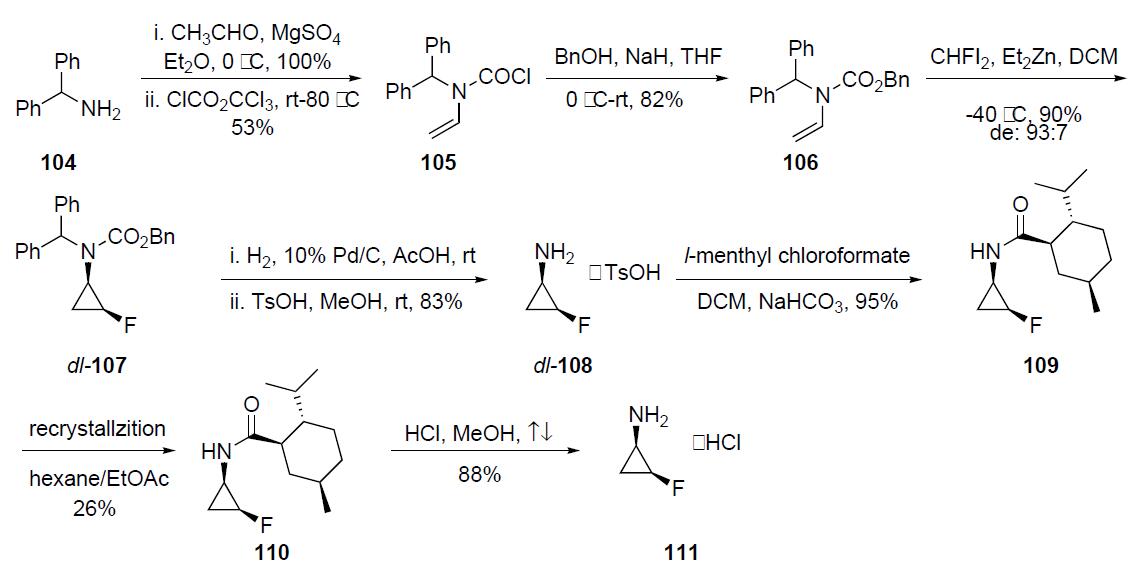
| | Mode of action |
Sitafloxacin inhibits DNA gyrase and topoisomerase IV. These enzymes are involved in bacteria DNA replication, transcription, repair, and recombination. Sitafloxacin has less activity against human topoisomerase II, an enzyme involved in cell growth. According to Akasaka et al. DNA gyrase, consisting of the subunits GyrA and GyrB, has ATP-dependent DNA supercoil-ing activity and is a primary target of quinolones in Gram-negative species, such as Escherichia coli and Neisseria gonorrhoeae. In contrast, topoisomerase IV, consisting of the subunits ParC and ParE, has an essential role in partitioning replicated chromosomes and is more sensitive than DNA gyrase to some quinolones, such as levofloxacin and ciprofloxacin in the Gram-positive species, such as Staphylococcus aureus and Streptococcus pneumoniae[1].
| | References | [1] Ghebremedhin, B. “Bacterial Infections in the Elderly Patient: Focus on Sitafloxacin.” 2012. 0. |
| | Sitafloxacin Sesquihydrate Preparation Products And Raw materials |
|