|
|
| | 1-BOC-5-CYANOINDOLE Basic information |
| Product Name: | 1-BOC-5-CYANOINDOLE | | Synonyms: | N-Boc-5-cyanoindole;N-(tert-Butoxycarbonyl)-5-cyanoindole;1-BOC-5-CYANOINDOLE;tert-Butyl 5-cyano-1H-indole-1-carboxylate;5-cyano-1-indolecarboxylic acid tert-butyl ester;1H-Indole-1-carboxylicacid, 5-cyano-, 1,1-dimethylethyl este...;5-Cyano-indole-1-carboxylic acid tert-butyl ester;1-BOC-5-Cyano-1H-indole | | CAS: | 475102-10-4 | | MF: | C14H14N2O2 | | MW: | 242.27 | | EINECS: | | | Product Categories: | blocks;Carboxes;IndolesOxindoles;Indole | | Mol File: | 475102-10-4.mol | 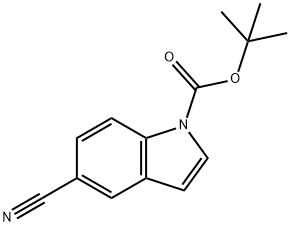 |
| | 1-BOC-5-CYANOINDOLE Chemical Properties |
| Melting point | 128-129 °C(Solv: ethanol (64-17-5); hexane (110-54-3)) | | Boiling point | 386.4±34.0 °C(Predicted) | | density | 1.12±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | Appearance | White to off-white Solid |
| | 1-BOC-5-CYANOINDOLE Usage And Synthesis |
| Synthesis | In a 100 mL round bottom flask, 5-cyanoindole (2.0 g, 14.07 mmol) was dissolved in 20 mL of anhydrous tetrahydrofuran (THF). To this solution, 4-dimethylaminopyridine (DMAP, 0.86 g, 7.03 mmol) was added and the mixture was stirred for 0.5 h at room temperature. Subsequently, di-tert-butyl dicarbonate (Boc2O, 3.07 g, 14.07 mmol) was added and the reaction continued to be stirred for 2 hours. After completion of the reaction, the reaction was quenched with water and extracted with ether (2×). The organic layers were combined, washed sequentially with 1N hydrochloric acid, water and saturated brine, dried over anhydrous magnesium sulfate (MgSO4) and concentrated to give 1-Boc-5-cyanoindole (3.26 g, 96% yield) as a white solid. The product was characterized by 1H NMR (DMSO-d6): δ 8.20-8.14 (m, 2H), 7.83 (d, 1H), 7.70 (d, 1H), 6.80 (d, 1H), 1.63 (s, 9H). | | References | [1] Synthesis, 2009, # 21, p. 3617 - 3632
[2] Journal of Organic Chemistry, 2002, vol. 67, # 21, p. 7551 - 7552
[3] Patent: WO2004/43950, 2004, A1. Location in patent: Page 134
[4] Patent: WO2005/51957, 2005, A1. Location in patent: Page/Page column 54-55
[5] Patent: WO2011/60389, 2011, A1. Location in patent: Page/Page column 98-99 |
| | 1-BOC-5-CYANOINDOLE Preparation Products And Raw materials |
|