|
|
| | Methyl 3,5-dimethoxybenzoate Basic information |
| Product Name: | Methyl 3,5-dimethoxybenzoate | | Synonyms: | RARECHEM AL BF 0065;METHYL 3,5-DIMETHOXYBENZOATE;Methyl 3,6-Dimethoxy Benzoate;3,5-DIMETHOXYBENZOIC ACID METHYL ESTER;Ethyl 3,5-dimethoxybenzoate 99%;Methyl 3,5-dimethoxybenzoate, 98+%;Benzoic acid, 3,5-dimethoxy-, methyl ester;3,5-Dimethoxybenzoic acid methyl | | CAS: | 2150-37-0 | | MF: | C10H12O4 | | MW: | 196.2 | | EINECS: | 218-423-6 | | Product Categories: | Building Blocks for Dendrimers;Functional Materials;C10 to C11;Carbonyl Compounds;Esters;Aromatic Esters;bc0001 | | Mol File: | 2150-37-0.mol | 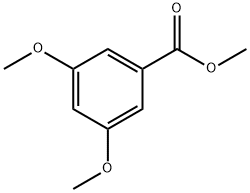 |
| | Methyl 3,5-dimethoxybenzoate Chemical Properties |
| Melting point | 42-43 °C (lit.) | | Boiling point | 298 °C (lit.) | | density | 1.2166 (rough estimate) | | refractive index | 1.5430 (estimate) | | Fp | >230 °F | | storage temp. | Sealed in dry,Room Temperature | | solubility | almost transparency in Methanol | | form | powder to lump to clear liquid | | color | White or Colorless to Light yellow | | BRN | 2693126 | | InChI | InChI=1S/C10H12O4/c1-12-8-4-7(10(11)14-3)5-9(6-8)13-2/h4-6H,1-3H3 | | InChIKey | YXUIOVUOFQKWDM-UHFFFAOYSA-N | | SMILES | C(OC)(=O)C1=CC(OC)=CC(OC)=C1 | | LogP | 1.850 (est) | | CAS DataBase Reference | 2150-37-0(CAS DataBase Reference) | | NIST Chemistry Reference | Benzoic acid, 3,5-dimethoxy-, methyl ester(2150-37-0) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 24/25-36/37-26 | | WGK Germany | 3 | | HS Code | 29189900 | | Storage Class | 11 - Combustible Solids |
| | Methyl 3,5-dimethoxybenzoate Usage And Synthesis |
| Description | Methyl 3,5-dimethoxybenzoate is a benzoate analogue mainly used in the field of organic synthesis. It can be used to prepare compounds such as 5,7-Dimethoxyindan-1-one and 3,5-Dimethoxyphenylacetonitrile. | | Chemical Properties | white to beige powder or granules | | Uses | Methyl 3,5-dimethoxybenzoate was used as starting reagent in the synthesis of 5,7-dimethoxy-4-methylphthalide, a key intermediate in the synthesis of mycophenolic acid and its analogues. | | General Description | Bromination of methyl 3,5-dimethoxybenzoate has been investigated. |
| | Methyl 3,5-dimethoxybenzoate Preparation Products And Raw materials |
|