| Company Name: |
Enzo Biochem Inc
|
| Tel: |
Enzo Biochem Inc. 13797054060 |
| Email: |
Enzoname@qq.com |
| Products Intro: |
Product Name:Lorcainide . HCl
|
|
| | N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride Basic information |
| Product Name: | N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride | | Synonyms: | N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride;LORCAINIDE HYDROCHLORIDE;Isocainide hydrochloride;4'-Chloro-N-(1-isopropyl-4-piperidyl)-2-phenylacetanilide monohydrochloride;Benzeneacetamide, N-(4-chlorophenyl)-N-[1-(1-methylethyl)-4-piperidinyl]-, monohydrochloride;Nsc310411;N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride USP/EP/BP;Lorcainide HCl | | CAS: | 58934-46-6 | | MF: | C22H28Cl2N2O | | MW: | 407.37652 | | EINECS: | 261-504-6 | | Product Categories: | | | Mol File: | 58934-46-6.mol | 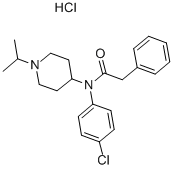 |
| | N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride Chemical Properties |
| Melting point | 263° | | storage temp. | -20°C | | solubility | Soluble in DMSO (up to 20 mg/ml), in Water (up to 20 mg/ml), or in Ethanol (up to 20 mg/ml). | | form | White solid. | | color | Powder | | Water Solubility | H2O: 10mg/mL, clear | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO, distilled water or ethanol may be stored at -20°C for up to 1 month | | InChI | 1S/C22H27ClN2O.ClH/c1-17(2)24-14-12-21(13-15-24)25(20-10-8-19(23)9-11-20)22(26)16-18-6-4-3-5-7-18;/h3-11,17,21H,12-16H2,1-2H3;1H | | InChIKey | FSFSWNBDCJVFGI-UHFFFAOYSA-N | | SMILES | Clc1ccc(cc1)N(C3CCN(CC3)C(C)C)C(=O)Cc2ccccc2.Cl |
| WGK Germany | WGK 3 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Acute Tox. 4 Oral |
| | N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride Usage And Synthesis |
| Description | Lorcainide hydrochloride (58934-46-6) is a voltage-gated Na+channel blocker.1Protects murine cultured cortical neurons from injury induced by oxygen-glucose deprivation.2Acts at the ouabain binding site of guinea pig cardiac Na+/K+ATPase and inhibits the enzyme (IC50= 34 μM).3 | | Uses | Cardiac depressant (anti-arrhythmic). | | References | [1] R S SHELDON H J D R J Hill. Antiarrhythmic drugs and the cardiac sodium channel: current models.[J]. Clinical chemistry, 1989, 35 5: 748-754.
[2] J J LYNCH. Sodium channel blockers reduce oxygen-glucose deprivation-induced cortical neuronal injury when combined with glutamate receptor antagonists.[J]. Journal of Pharmacology and Experimental Therapeutics, 1995, 273 1: 554-560.
[3] A. ALMOTREFI. Class I antiarrhythmic drug effects on ouabain binding to guinea pig cardiac Na+ -K+ ATPase.[J]. Canadian journal of physiology and pharmacology, 1999, 77 11 1: 866-870. DOI:10.1139/y99-091 |
| | N-(4-chlorophenyl)-N-(1-isopropyl-4-piperidyl)phenylacetamide monohydrochloride Preparation Products And Raw materials |
|