(1R,2S)-2-fluorocyclopropanecarboxylic acid manufacturers
|
| | (1R,2S)-2-fluorocyclopropanecarboxylic acid Basic information |
| Product Name: | (1R,2S)-2-fluorocyclopropanecarboxylic acid | | Synonyms: | (1R,2S)-2-fluorocyclopropanecarboxylic acid;Cyclopropanecarboxylic acid, 2-fluoro-, (1R-trans)- (9CI);(1R,2S)-2-fluorocyclopropane-1-carboxylic acid;(1R,2S)-trans-2-Fluorocyclopropanecarboxylic acid 97%;Sitafloxacin Impurity 26 | | CAS: | 167073-08-7 | | MF: | C4H5FO2 | | MW: | 104.08 | | EINECS: | | | Product Categories: | | | Mol File: | 167073-08-7.mol | 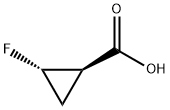 |
| | (1R,2S)-2-fluorocyclopropanecarboxylic acid Chemical Properties |
| Boiling point | 202.3±33.0 °C(Predicted) | | density | 1.35±0.1 g/cm3(Predicted) | | storage temp. | Store at room temperature | | pka | 4.00±0.11(Predicted) | | Appearance | White to yellow Solid | | Optical Rotation | 44.001° (C=0.01 g/100ml, CHCL3 ) | | InChI | InChI=1S/C4H5FO2/c5-3-1-2(3)4(6)7/h2-3H,1H2,(H,6,7)/t2-,3-/m0/s1 | | InChIKey | HZQKMZGKYVDMCT-HRFVKAFMSA-N | | SMILES | [C@H]1(C(O)=O)C[C@@H]1F |
| | (1R,2S)-2-fluorocyclopropanecarboxylic acid Usage And Synthesis |
| Synthesis |
Dissolve benzyl alcohol and allyl bromide in the solvent, add sodium hydride, and perform a condensation reaction to obtain allyl benzyl ether. The obtained allyl benzyl ether, dichlorofluoromethane, and sodium hydroxide solution are raw materials for carbene reaction to obtain 2-chloro-2-fluorocyclopropylmethyl benzyl ether. Subsequently, the obtained 2-chloro-2-fluorocyclopropylmethylbenzyl ether is hydrogenated and reduced to obtain 2-chloro-2-fluorocyclopropanemethanol. Sodium hypochlorite is added as an oxidizing agent to perform an oxidation reaction to obtain 2-chloro-2-fluorocyclopropanecarboxylic acid. Finally, diethylamine is added to perform a hydrogenation reduction reaction to obtain (1R,2S)-2-fluorocyclopropanecarboxylic acid.

|
| | (1R,2S)-2-fluorocyclopropanecarboxylic acid Preparation Products And Raw materials |
|