|
|
| | 5'-CHLORO-5'-DEOXYADENOSINE Basic information |
| Product Name: | 5'-CHLORO-5'-DEOXYADENOSINE | | Synonyms: | 5'-CHLORO-5'-DEOXYADENOSINE;5-CHLORO-5-DEOXYADENOSINE HYDRATE;5'-CHLORO-5'-DEOXYADENOSINE MONOHYDRATE;5’-chloro-5’-deoxy-adenosin;5'-CHLORO-5'-DEOXY-D-ADENOSINE;5'-Deoxy-5'-chloroadenosine;5'-Cl-5'-deoxyadenosine;Adenosine,5'-chloro-5'-deoxy- | | CAS: | 892-48-8 | | MF: | C10H12ClN5O3 | | MW: | 285.69 | | EINECS: | | | Product Categories: | | | Mol File: | 892-48-8.mol | 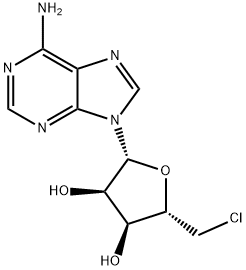 |
| | 5'-CHLORO-5'-DEOXYADENOSINE Chemical Properties |
| Melting point | 187 °C (exothermic decomp onset @144.5 C)(lit.) | | Boiling point | 645.6±65.0 °C(Predicted) | | density | 2.03±0.1 g/cm3(Predicted) | | storage temp. | Keep in dark place,Sealed in dry,2-8°C | | solubility | DMSO (Slightly), Water (Slightly, Heated) | | form | Solid | | pka | 13.03±0.70(Predicted) | | color | Off-White to Light Beige | | InChI | InChI=1S/C10H12ClN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h2-4,6-7,10,17-18H,1H2,(H2,12,13,14)/t4-,6-,7-,10-/m1/s1 | | InChIKey | IYSNPOMTKFZDHZ-KQYNXXCUSA-N | | SMILES | C(Cl)[C@H]1O[C@@H](N2C3C(=C(N=CN=3)N)N=C2)[C@H](O)[C@@H]1O |
| WGK Germany | 3 | | RTECS | AU7357200 |
| | 5'-CHLORO-5'-DEOXYADENOSINE Usage And Synthesis |
| Uses | 5''-Deoxy-5''-chloroadenosine is an intermediate in the synthesis of 5''-Deoxy-5''-(methylthio)adenosine (D242600), a substrate to study the specificity and kinetics of 5′-methylthioadenosinephosphorylase (MTAP) (EC2.4.2.28), a tumor suppressor gene expressed enzyme that supports the S-adenosylmethionine (AdoMet) and methionine salvage pathways. | | Definition | ChEBI: 5'-chloro-5'-deoxyadenosine is a member of adenosines. It is functionally related to an adenosine. | | Synthesis | General steps:
1. Preparation of (2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-(chloromethyl) tetrahydrofuran-3,4-diol
Under ice bath cooling conditions, (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (1 g, 3.47 mmol) was dissolved in acetonitrile (10 ml), pyridine (593 mg, 0.6 ml, 7.49 mmol) and SOCl2 (2.22 g, 1.36 ml, 18.65 mmol). The reaction mixture was stirred at 0-5 °C for 3-4 h, followed by slow warming to room temperature and stirring overnight. After completion of the reaction, the suspension was concentrated in vacuum. Methanol (20 ml), water (2 ml) and NH4OH (4 ml) were added to the concentrate and stirred for 0.5 h at room temperature. The reaction mixture was again concentrated to dryness. The crude product was dissolved in methanol, silica gel (3 g) was added and the solvent was subsequently removed. The residue was purified by silica gel column chromatography (SGC) using gradient elution with ethyl acetate:methanol (0%-10%) to afford the target compound (0.915 g, 86% yield) as a light yellow solid.
NMR (500 MHz, MeOD): δ 8.24 (s, 1H), 8.02 (s, 1H), 7.27-7.20 (m, 5H), 6.19 (d, J = 2.0 Hz, 1H), 5.48-5.46 (m, 1H), 5.08-5.06 (m, 1H), 4.43 (t, J = 4.0 Hz, 1H), 3.83 -3.81 (m, 2H), 3.01-2.99 (br s, 2H), 1.60 (s, 3H), 1.38 (s, 3H) ppm.
LCMS (m/z): 395.8 [M + H]+. | | References | [1] Journal of Medicinal Chemistry, 2012, vol. 55, # 18, p. 8066 - 8074,9
[2] Angewandte Chemie - International Edition, 2016, vol. 55, # 46, p. 14277 - 14280
[3] Angew. Chem., 2016, vol. 128, # 46, p. 14489 - 14492,4
[4] Tetrahedron Letters, 2018, vol. 59, # 4, p. 415 - 417
[5] Patent: US4373097, 1983, A |
| | 5'-CHLORO-5'-DEOXYADENOSINE Preparation Products And Raw materials |
|