反式-1-(4-羟基环己基)-4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)咪唑
| 中文名称 | 反式-1-(4-羟基环己基)-4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)咪唑 |
|---|---|
| 中文同义词 | 反式-1-(4-羟基环己基)-4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)咪唑;反-4-[4-(4-氟苯基)-5-(2-甲氧基-4-嘧啶基)-1H-咪唑-1-基]环己醇;(1R,4R)-REL-4-(4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)-1H-咪唑-1-基)环己醇;化合物L-779450;化合物SB239063,10 MM DMSO 溶液;SB 239063试剂;SB 239063 (SB-239063;SB239063);SB 239063试剂 |
| 英文名称 | SB 239063 |
| 英文同义词 | SB 239063;TRANS-1-(4-HYDROXYCYCLOHEXYL)-4-(4-FLUOROPHENYL)-5-(2-METHOXYPYRIDIMIDIN-4-YL)IMIDAZOLE;TRANS-1-(4-HYDROXYCYCLOHEXYL)-4-(4-FLUOROPHENYL)-5-(2-METHOXYPYRIMIDIN-4-YL) IMIDAZOLE;TRANS-1-(4-HYDROXYCYCLOHEXYL)-4-(FLUOROPHENYL)-5-(2-METHOXYPYRIMIDIN-4-YL) IMIDAZOLE;TRANS-4-[4-(4-FLUOROPHENYL)-5-(2-METHOXY-4-PYRIMIDINYL)-1H-IMIDAZOL-1-YL]CYCLOHEXANOL;Cyclohexanol, 4-[4-(4-fluorophenyl)-5-(2-methoxy-4-pyrimidinyl)-1H-imidazol-1-yl]-, trans-;CS-402;SB-239063; SB239063 |
| CAS号 | 193551-21-2 |
| 分子式 | C20H21FN4O2 |
| 分子量 | 368.4 |
| EINECS号 | |
| 相关类别 | Aromatics;Heterocycles;Protein Kinase Inhibitors and Activators |
| Mol文件 | 193551-21-2.mol |
| 结构式 | 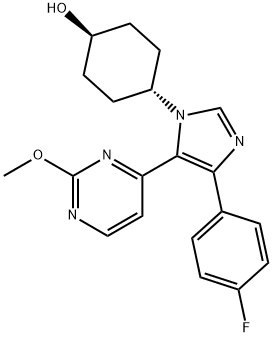 |
反式-1-(4-羟基环己基)-4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)咪唑 性质
| 熔点 | 206-207.2 °C |
|---|---|
| 储存条件 | 2-8°C |
| 溶解度 | 二甲基亚砜:11 毫克/毫升 |
| 形态 | 白色固体 |
| 颜色 | 白色 |
| InChI | InChI=1S/C20H21FN4O2/c1-27-20-22-11-10-17(24-20)19-18(13-2-4-14(21)5-3-13)23-12-25(19)15-6-8-16(26)9-7-15/h2-5,10-12,15-16,26H,6-9H2,1H3/t15-,16- |
| InChIKey | ZQUSFAUAYSEREK-WKILWMFISA-N |
| SMILES | [C@@H]1(O)CC[C@@H](N2C=NC(C3=CC=C(F)C=C3)=C2C2C=CN=C(OC)N=2)CC1 |
| Target | Value |
|
p38α
(Cell-free assay) | 44 nM |
|
p38β
(Cell-free assay) | 44 nM |
SB 239063 (0.1–10 μM ; 29 hours, 47 hours) increases apoptosis of eosinophils in a dose-related in the presence of 10 pM IL-5 at every time point from 21 hours onwards.
SB 239063 potently inhibits IL-1 and TNF- a production in LPS-stimulated human peripheral blood monocytes with IC
50
values of 120 nM and 350 nM, respectively.
Apoptosis Analysis
| Cell Line: | Eosinophils (guinea pig BALs) |
| Concentration: | 0.1μM, 1μM, 10μM |
| Incubation Time: | 29 hours, 47 hours |
| Result: | Increased apoptosis of eosinophils in a dose-related in the presence of 10 pM IL-5 at every time point from 21 hours onwards. |
SB 239063 (12 mg/kg; p.o.; 1 hour before and 4 hours after OA challenge; b.i.d. for 3 days) significantly inhibits the resultant antigen-induced airway eosinophilia.
SB 239063 (12 mg/kg; p.o.) almost abolishes ovalbumin (OA)-induced airway eosinophilia (~ 93% inhibition) by inhalation.
SB 239063 is a potent inhibitor of LPS-induced TNF-alpha production in the mouse peritoneal cavity with an EC
50
of 5.8 mg/kg (2.8–10.3; 95% CL).
| Animal Model: | Male BALB/c mice (18–20 g) |
| Dosage: | 12 mg/kg |
| Administration: | Oral administration; 1 h before and 4 h after OA challenge; bis in die for 3 days |
| Result: | Significantly inhibited the resultant antigen-induced airway eosinophilia. |
![Cyclohexanone, 4-[4-(4-fluorophenyl)-5-(2-methoxy-4-pyrimidinyl)-1H-imidazol-1-yl]-](/CAS/20210305/GIF/193551-20-1.gif)
193551-20-1

193551-21-2
以化合物(CAS: 193551-20-1)为起始原料,合成(1r,4r)-rel-4-(4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)-1H-咪唑-1-基)环己醇的一般步骤如下: 实施例2:反式-1-(4-羟基环己基)-4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)咪唑的合成 向实施例1(j)中制备的化合物溶液(0.099 g,溶解于MeOH/THF(1 mL,体积比1:1)中,对应0.27 mmol)加入NaBH4溶液(1 mL,1 M溶液。该NaBH4溶液通过将0.10 g NaBH4、MeOH(2.5 mL)和25% NaOMe的MeOH溶液(0.2 mL)混合制备而成)。反应混合物搅拌10分钟后,用饱和Na2CO3溶液淬灭反应,随后蒸发除去溶剂。残余物通过从MeOH/H2O混合溶剂中重结晶纯化,得到目标产物(1r,4r)-rel-4-(4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)-1H-咪唑-1-基)环己醇,为白色针状晶体(0.063 g,收率63%)。产物的熔点为188-190℃。
参考文献:
[1] Patent: US6329526, 2001, B1
安全信息
| 安全说明 | 22-24/25 |
|---|---|
| WGK Germany | 3 |
| RTECS号 | GV9458000 |
| 海关编码 | 2933599590 |
| 存储类别 | 11 - 可燃固体 |
| 提供商 | 语言 |
|---|---|
|
英文
|
| 更新日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
|---|---|---|---|---|---|
| 2025/12/22 | B5898 | SB 239063 SB 239063 | 193551-21-2 | 5mg | 350元 |
| 2025/12/22 | B6671 | 反式-1-(4-羟基环己基)-4-(4-氟苯基)-5-(2-甲氧基嘧啶-4-基)咪唑 SB 239063 [Optimized for Cell Culture] | 193551-21-2 | 1MG | 390元 |
