
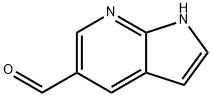
(1H-PYRROLO[2,3-B]PYRIDIN-5-YL)-METHANOL synthesis
- Product Name:(1H-PYRROLO[2,3-B]PYRIDIN-5-YL)-METHANOL
- CAS Number:849067-97-6
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
![1H-PYRROLO[2,3-B]PYRIDINE-5-CARBOXYLIC ACID METHYL ESTER](/CAS/GIF/849067-96-5.gif)
849067-96-5
![(1H-PYRROLO[2,3-B]PYRIDIN-5-YL)-METHANOL](/CAS/GIF/849067-97-6.gif)
849067-97-6
General procedure for the synthesis of (1H-pyrrolo[2,3-b]pyridin-5-yl)methanol from methyl 1H-pyrrolo[2,3-b]pyridine-5-carboxylate: Step C: Preparation of (1H-pyrrolo[2,3-b]pyridin-5-yl)methanol [0249] Methyl 1H-pyrrolo[2,3-b]pyridine-5-carboxylate (75 mg, 0.394 mmol) was dissolved in THF at 0 °C and lithium aluminum hydride (45 mg, 1.18 mmol) was added slowly. The reaction mixture was gradually warmed to room temperature followed by refluxing for 12 hours. After completion of the reaction, it was cooled to room temperature and quenched with water. The reaction mixture was extracted with ethyl acetate, the organic phase was washed with brine, dried over anhydrous sodium sulfate and concentrated to give (1H-pyrrolo[2,3-b]pyridin-5-yl)methanol (57 mg, 98% yield). Product characterization data: 1H NMR (500 MHz, DMSO-d6) δ: 11.50 (1H, bs), 8.166-8.163 (1H, d), 7.862-7.860 (1H, d), 7.43-7.42 (1H, d), 6.41-6.40 (1H, d), 5.10-5.08 (1H, t), 4.58-4.57 ( 2H, d). FIA MS, m/z [MH]+ 149.1.
849067-90-9
102 suppliers
$85.00/250mg
![(1H-PYRROLO[2,3-B]PYRIDIN-5-YL)-METHANOL](/CAS/GIF/849067-97-6.gif)
849067-97-6
73 suppliers
$65.00/50mg
Yield: 76%
Reaction Conditions:
with methanol;sodium tetrahydroborate for 1 h;
Steps:
93
To the solution of lH-pyrrolo[2,3-b]pyridine-5-carbaldehyde (400 mg, 2.73 mmol, 1.0 eq) in MeOH (10 mL) was added sodium borohydride (208 mg, 5.46 mmol, 2.0 eq). The reaction was stirred for 1 h, and then purified via flash chromatography to afford (lH-pyrrolo[2,3-b]pyridin-5-yl)methanol as a white solid (310 mg, 76%).
References:
LIFESCI PHARMACEUTICALS, INC.;MCDONALD, Andrew WO2015/103317, 2015, A1 Location in patent:Page/Page column 175
![1H-PYRROLO[2,3-B]PYRIDINE-5-CARBOXYLIC ACID METHYL ESTER](/CAS/GIF/849067-96-5.gif)
849067-96-5
113 suppliers
$23.00/250mg
![(1H-PYRROLO[2,3-B]PYRIDIN-5-YL)-METHANOL](/CAS/GIF/849067-97-6.gif)
849067-97-6
73 suppliers
$65.00/50mg
![1H-PYRROLO[2,3-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/754214-42-1.gif)
754214-42-1
149 suppliers
$20.00/100mg
![(1H-PYRROLO[2,3-B]PYRIDIN-5-YL)-METHANOL](/CAS/GIF/849067-97-6.gif)
849067-97-6
73 suppliers
$65.00/50mg