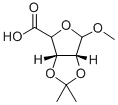
2,3-O-ISOPROPYLIDENE-1-O-METHYL-D-RIBOSIC ACID synthesis
- Product Name:2,3-O-ISOPROPYLIDENE-1-O-METHYL-D-RIBOSIC ACID
- CAS Number:54622-95-6
- Molecular formula:C9H14O6
- Molecular Weight:218.2

4099-85-8

54622-95-6
General procedure for the synthesis of 2,3-o isopropylidene-1-o-methyl-d-ribonucleic acid from (3aR,4R,6R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxydan-4-yl)methanol: At 0 °C, KBr (1.70 g, 14.7 mmol) and TEMPO (0.383 g 2.45 mmol) were added to a solution of (3aR,4R,6R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol (10.0 g, 49.0 mmol) in EtOAc (100 mL), followed by the titrative addition of NaOCl (12%, 39.5 mL. 63.7 mmol). The reaction solution was adjusted to pH 2 with 35% HCl, followed by the addition of 25% aqueous NaCl (5.76 g, 63.7 mmol) over 30 min, keeping stirring at room temperature. The reaction lasted for 7 h and was completed. The pH was adjusted to 2-3 and the product was extracted with EtOAc. The organic phases were combined, washed with brine, dried over MgSO4, filtered and the solvent was concentrated under reduced pressure to give the crude product. The crude product was purified by recrystallization from n-hexane in hot CH2Cl2 to give a white solid; Yield: 10.68 g (quantitative); Melting point: 128.5-129.5 °C (literature value 27b: 129-131 °C); [α]D20.5 -67.29 (c 2.47, CHCl3).1H NMR (CDCl3, 400 MHz): δ= 10.69 (s, 1H), 5.20 (d, J = 6.3Hz, 1H), 5.09 (s, 1H), 4.69 (s, 1H), 4.59 (d, J = 6.0Hz, 1H), 3.45 (s, 3H), 1.50 (s, 3H), 1.34 (s, 3H).

4099-85-8
141 suppliers
$45.00/5 g

54622-95-6
71 suppliers
$60.00/100mg
Yield: 100%
Reaction Conditions:
Stage #1:((3aR,4R,6R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol with sodium hypochlorite;tetrabutylammomium bromide;sodium bromide;2,2,6,6-tetramethyl-piperidine-N-oxyl;sodium hydrogencarbonate in dichloromethane at 0 - 20; for 1 h;
Stage #2: with sodium chlorite;sodium dihydrogenphosphate;2-methyl-but-2-ene in tetrahydrofuran;water;tert-butyl alcohol at 20;Further stages.;
References:
Huang, Lijun;Teumelsan, Nardos;Huang, Xuefei [Chemistry - A European Journal,2006,vol. 12,# 20,p. 5246 - 5252]