
7-Nitroindazole synthesis
- Product Name:7-Nitroindazole
- CAS Number:2942-42-9
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13

570-24-1

2942-42-9
General procedure for the synthesis of 7-nitroindazole from 2-methyl-6-nitroaniline: To a stirred solution of 2-methyl-6-nitroaniline (10 g, 0.065 mol) in acetic acid (470 ml, 47 times the volume) was slowly added an aqueous solution (9 ml) of sodium nitrite (1.54 g, 0.06 mol, 1.1 equiv). The reaction mixture was stirred at room temperature for 30-45 min. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent 30% ethyl acetate/hexane, Rf=0.7). After completion of the reaction, acetic acid was removed by distillation under reduced pressure. The obtained residue was dissolved in ice water (200 ml), the precipitated solid was filtered, washed with cold water and dried under vacuum to afford the target product 7-nitroindazole as a yellow solid (10 g, 98% yield).

570-24-1
361 suppliers
$6.00/10g

2942-42-9
260 suppliers
$5.00/100mg
Yield:2942-42-9 98%
Reaction Conditions:
with acetic acid;sodium nitrite in water;
Steps:
6.6.14.d
To a stirred solution of step-a-c product (10 g, 0.065 mol) in acetic acid (470 ml, 47 times), a solution of sodium nitrite (1.54 g, 0.06 mol, 1.1 eq) in water (9 ml) was added and the reaction mass was stirred for 30 - 45 min. Progress of the reaction was monitored by TLC (30% ethyl acetate/hexane, Rr-0.7). On completion of the reaction, acetic acid was distilled off and the residue obtained was taken in ice water (200 ml). Solid formed was filtered, washed with cold water and dried to yield the required product as an yellow colored solid (10 g, 98% yield).
References:
WO2010/127855,2010,A1 Location in patent:Page/Page column 124; 125

173459-52-4
11 suppliers
inquiry

2942-42-9
260 suppliers
$5.00/100mg
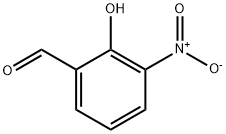
5274-70-4
206 suppliers
$34.00/1g

2942-42-9
260 suppliers
$5.00/100mg