
tert-butyl 6-methoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate synthesis
- Product Name:tert-butyl 6-methoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate
- CAS Number:860436-57-3
- Molecular formula:C15H21NO3
- Molecular Weight:263.33
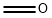
50-00-0

24424-99-5

2039-67-0

860436-57-3
1. 3-Methoxyphenethylamine (1.0 eq.) and MgSO4 (3.0 eq.) were suspended in anhydrous CH2Cl2, followed by batch addition of paraformaldehyde (5.0 eq.). After stirring for 2 hours, the solids were removed by filtration and the filtrate was concentrated. 2. The residue was dissolved in anhydrous trifluoroacetic acid and refluxed overnight under nitrogen protection. After completion of the reaction, the mixture was poured into an ice-water mixture, the aqueous phase was adjusted to alkaline with 6 M NaOH and extracted with CH2Cl2. The organic phase was dried over MgSO4 and concentrated. 3. The remaining oil was dissolved in anhydrous DMF and di-tert-butyl dicarbonate (1.2 eq.) and triethylamine (3.0 eq.) were added. The mixture was stirred for 3 h and concentrated, the residue was dissolved in ethyl acetate and washed with saturated Na2CO3 solution. The organic phase was dried over MgSO4 and concentrated. 4. Purification by fast column chromatography (silica gel, gradient elution, petroleum ether solution with 10-30% EtOAc) afforded tert-butyl 6-methoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate in 47% yield. 5. Concentrated HNO3 (1.5 eq.) was added to acetic anhydride (6 eq.) at -20°C under nitrogen protection, and the solution was subsequently added to a nitromethane solution of tert-butyl 6-methoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate. The reaction mixture was stirred at -20°C for 3 hours before toluene was added, washed with saturated NaHCO3 solution and brine, dried (MgSO4) and concentrated. 6. Purification by fast column chromatography (silica gel, petroleum ether solution with gradient elution of 20-50% EtOAc) gives a 1:1 mixture of nitration products 3a and 3b in 66% yield. 7. The amine 3a or 3b was dissolved in 48% HBr aqueous solution, refluxed for 12 h and concentrated to dryness to quantitatively obtain the hydrobromide salt of 4a or 4b. 8. Amine 4a or 4b (1 eq.) was dissolved in anhydrous DMF, triethylamine (3 eq.) was added, stirred for 15 min and then 2-(4-chlorophenyl)ethyl isothiocyanate (1.2 eq.) was added. The mixture was stirred for 5 h and concentrated, the residue was dissolved in EtOAc, washed with water, the organic phase was dried (MgSO4) and concentrated. 9. Purification by fast column chromatography (silica gel, petroleum ether:EtOAc (3:2)) afforded N-[2-(4-chlorophenyl)ethyl]-6-hydroxy-7-nitro-3,4-dihydroisoquinoline-2(1H)-thiocarboxamide (Res-14-54) in 76% yield or purification by (silica gel, CH2Cl2:petroleum ether (8:2)) afforded N-[ 2-(4-chlorophenyl)ethyl]-6-hydroxy-5-nitro-3,4-dihydroisoquinoline-2(1H)-carbothioamide (Res-14-56) in 40% yield.

42923-77-3
143 suppliers
$21.00/100mg

24424-99-5
860 suppliers
$13.50/25G

860436-57-3
24 suppliers
inquiry
Yield:860436-57-3 66%
Reaction Conditions:
in dichloromethane at 20; for 2 h;
References:
Gini, Andrea;Bamberger, Julia;Luis-Barrera, Javier;Zurro, Mercedes;Mas-Ballesté, Rubén;Alemán, José;Manche?o, Olga García [Advanced Synthesis and Catalysis,2016,vol. 358,# 24,p. 4049 - 4056] Location in patent:supporting information

42923-77-3
143 suppliers
$21.00/100mg

24608-52-4
19 suppliers
inquiry

860436-57-3
24 suppliers
inquiry
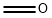
50-00-0
897 suppliers
$10.00/25g

24424-99-5
860 suppliers
$13.50/25G

2039-67-0
240 suppliers
$17.47/5G

860436-57-3
24 suppliers
inquiry

24424-99-5
860 suppliers
$13.50/25G

57196-62-0
142 suppliers
$9.00/250mg

860436-57-3
24 suppliers
inquiry

2039-67-0
240 suppliers
$17.47/5G

860436-57-3
24 suppliers
inquiry