
Methyl 5-bromo-6-chloropyridine-3-carboxylate synthesis
- Product Name:Methyl 5-bromo-6-chloropyridine-3-carboxylate
- CAS Number:78686-77-8
- Molecular formula:C7H5BrClNO2
- Molecular Weight:250.48

29241-62-1
74-88-4

78686-77-8
Step 1: Synthesis of methyl 5-bromo-6-chloronicotinate (61). A mixture of 5-bromo-6-chloronicotinic acid (60) (0.2 g, 0.84 mmol), potassium carbonate (K2CO3) (0.3 g, 2.1 mmol) and iodomethane (0.178 g, 1.2 mmol) in N,N-dimethylformamide (DMF) (10 mL) was stirred for 16 h at 0 °C and subsequently raised to room temperature. Upon completion of the reaction, the reaction mixture was diluted with water (20 mL) and extracted with ethyl acetate (EtOAc) (2 x 20 mL). The organic phases were combined and washed sequentially with saturated aqueous sodium bicarbonate (NaHCO3) (20 mL) and brine (2 x 20 mL), dried over anhydrous sodium sulfate (Na2SO4) and concentrated to afford the target product methyl 5-bromo-6-chloronicotinate (61) (0.2 g, 94.7% yield).

29241-62-1
238 suppliers
$5.00/250mg
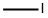
74-88-4
357 suppliers
$15.00/10g

78686-77-8
211 suppliers
$6.00/1g
Yield:78686-77-8 94.7%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
10.1 Step 1: Methyl 5-bromo-6-chloronicotinate (61).
Step 1: Methyl 5-bromo-6-chloronicotinate (61). A mixture of 5-bromo-6- chloronicotinic acid 60 (0.2 g, 0.84 mmol), K2C03 (0.3 g, 2.1 mmol) and methyl iodide (0.178 g, 1.2 mmol) in DMF (10 mL) was stirred for 16 h at RT. After completion, the reaction mixture was diluted with water (20 mL) and extracted with EtOAc (2 x 20 mL). The combined organic extracts were washed with saturated aqueous NaHC03 solution (20 mL), brine (2 x 20 mL) and dried (Na2S04) and concentrated to afford ester 61 (0.2 g, 94.7%).
References:
WO2017/4608,2017,A1 Location in patent:Paragraph 0183
381247-99-0
92 suppliers
$17.00/250mg

78686-77-8
211 suppliers
$6.00/1g

29241-62-1
238 suppliers
$5.00/250mg

18107-18-1
210 suppliers
$33.00/5g

78686-77-8
211 suppliers
$6.00/1g
67-56-1
790 suppliers
$9.00/25ml

78686-77-8
211 suppliers
$6.00/1g
67-56-1
790 suppliers
$9.00/25ml

29241-62-1
238 suppliers
$5.00/250mg

78686-77-8
211 suppliers
$6.00/1g