
Rabeprazole Sodium synthesis
- Product Name:Rabeprazole Sodium
- CAS Number:117976-90-6
- Molecular formula:C18H21N3O3S.Na
- Molecular Weight:382.43
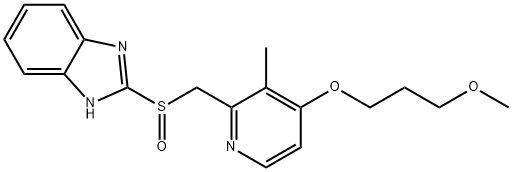
117976-89-3

117976-90-6
Example 2: Sodium hydroxide (2.25 g, 55.1 mmol) was dissolved in ethanol (30 ml) and stirred until completely dissolved. Subsequently, 2-(((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole (20.0 g, 55.6 mmol) was added to this solution and stirred until completely dissolved. After confirmation of dissolution, diisopropyl ether (2000 ml) was slowly added to induce precipitation of the target product 2-(((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methyl)sulfinyl)benzo[d]imidazole-1-ide sodium salt. The precipitate was collected by filtration to give 22.8 g of product in 100% yield. Example 3: Sodium hydroxide (1.65 g, 41.3 mmol) was dissolved in ethanol (22.5 ml) and stirred until completely dissolved. Then, 2-(((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole (15.0 g, 41.7 mmol) was added to this solution and stirred until completely dissolved. After confirmation of dissolution, tert-butyl methyl ether (160 ml) was slowly added to induce precipitation of the target product 2-(((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methyl)sulfinyl)benzo[d]imidazole-1-ide sodium salt. The precipitate was collected by filtration to give 15.9 g of product in 100% yield.

117976-89-3
244 suppliers
$15.00/1mg

117976-90-6
651 suppliers
$25.00/5mg
Yield:117976-90-6 100%
Reaction Conditions:
with sodium hydroxide in ethanolProduct distribution / selectivity;
Steps:
2; 3
Example 2 Ethanol (30 ml) was added to sodium hydroxide (2.25 g (55.1 mmol)), and dissolution was carried out. 2-[{4-(3-Methoxypropoxy)-3-methylpyridin-2-yl}methylsulfinyl]-1H-benzimidazole (20.0 g (55.6 mmol)) was then put into the ethanol solution, and after dissolution had been confirmed, diisopropyl ether (2000 ml) was added slowly, whereby 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylsulfinyl]-1H-benzimidazole sodium salt (22.8 g) was obtained through precipitation (yield 100%).; Example 3 Ethanol (22.5 ml) was added to sodium hydroxide (1.65 g (41.3 mmol)), and dissolution was carried out. 2-[{4-(3-Methoxypropoxy)-3-methylpyridin-2-yl}methylsulfinyl]-1H-benzimidazole (15.0 g (41.7 mmol)) was then put into the ethanol solution, and after dissolution had been confirmed, tert-butyl methyl ether (160 ml) was added slowly, whereby 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylsulfinyl]-1H-benzimidazole sodium salt (15.9 g) was obtained through precipitation (yield 100%).
References:
Urawa, Yoshio US2007/225502, 2007, A1 Location in patent:Page/Page column 5
![2-{[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl]methylthio}-1H-benzimidazole](/CAS/GIF/117977-21-6.gif)
117977-21-6
360 suppliers
$8.00/5g

117976-90-6
651 suppliers
$25.00/5mg
1310-73-2
1421 suppliers
$14.00/25g
![2-{[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl]methylthio}-1H-benzimidazole](/CAS/GIF/117977-21-6.gif)
117977-21-6
360 suppliers
$8.00/5g

117976-90-6
651 suppliers
$25.00/5mg
1310-73-2
1421 suppliers
$14.00/25g

117976-89-3
244 suppliers
$15.00/1mg

117976-90-6
651 suppliers
$25.00/5mg

117977-20-5
86 suppliers
inquiry

117976-90-6
651 suppliers
$25.00/5mg