| Structure |
Chemical Name |
CAS |
MF |
 |
Lithium carbonate (medicinal) |
|
|
 |
Medicinal wood activated carbon |
|
|
 |
Medicinal dipotassium hydrogen phosphate |
|
|
 |
Blank micropellets sucrose type, medicinal micropellets core |
|
|
 |
DryobalanopsaromaticaGwaertn.f. |
|
|
 |
Paraffin wax (SASOLWAX 2697) (medicinal excipients) |
|
|
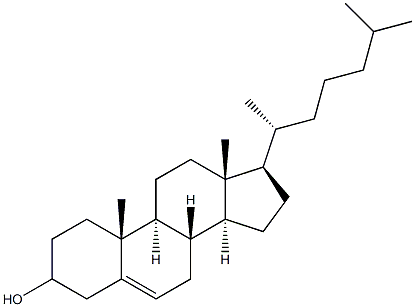 |
Cholesterol (for injection) (medicinal excipients) |
|
C27H46O |
 |
Carbomer 934P (medicinal excipients) |
|
|
 |
Lactose (medicinal excipients) |
|
|
 |
Glycolide lactide copolymer (5050) (medicinal excipients) |
|
|
 |
Pharmaceutical grade polysorbate 80 |
|
|
 |
Pharmaceutical grade polyethylene glycol 1000 |
|
|
 |
Triethanolamine (medicinal excipients) |
|
|
 |
Titanium dioxide (medicinal excipients) |
|
|
 |
Disodium edetate (medicinal excipients) |
|
|
 |
Sodium benzoate (medicinal excipients) |
|
|
 |
Glycerin (medicinal excipients) |
|
|
 |
Silica (SYLYSIA 350FCP) (medicinal excipients) |
|
|
 |
Methyl cellulose (medicinal excipients) |
|
|
 |
Film coating agent (enteric type) (medicinal excipients) |
|
|
 |
Starch soluble |
|
|
 |
Pharmaceutical film coating premix (stomach soluble) (medicinal excipients) |
|
|
 |
Pharmaceutical grade povidone K15 |
|
|
 |
Soybean phospholipid (medicinal excipient) |
|
|
 |
Croscarmellose sodium (medicinal excipients) |
|
|
 |
Potassium dihydrogen phosphate (medicinal excipients) |
|
|
 |
Medicinal pellets (starch type) (medicinal excipients) |
|
|
 |
Lactose (granular type) (medicinal excipients) |
|
C12H22O11 |
 |
Colloidal silica (medicinal excipients) |
|
|
 |
Polyethylene glycol 400 (for injection) (medicinal excipients) |
|
|
 |
Methanol (medicinal excipients) |
|
|
 |
Disodium edetate (for injection) (medicinal excipients) |
|
C10H14N2Na2O8 |
 |
Sulfobutyl betacyclodextrin sodium (medicinal excipients) |
|
|
 |
Ethyl acetate (medicinal excipients) |
|
|
 |
Emulsifier 400 do |
|
|
 |
Veronica officinalis, ext. |
85117-19-7 |
|
 |
Hydroxyethyl Cellulose |
|
|
 |
Salicylic acid Pharm grade |
|
C7H6O3 |
 |
Pharmaceutical ammonium acetate |
|
|
 |
Medicinal boric acid |
|
|
 |
Saccharin sodium (pharmaceutical excipient) |
|
|
 |
Macrogol 400 |
|
|
 |
Borax (pharmaceutical excipient) |
|
|
 |
Taurine (pharmaceutical excipient) |
|
|
 |
Pharmaceutical grade pregelatinized starch |
|
|
 |
Pharmaceutical grade sodium carboxymethyl starch |
|
|
 |
Medicinal iron powder |
|
|
 |
Medicinal Polysorbate 80 |
|
|
 |
Huang Dan for medicine |
|
|
 |
Medicinal solubilizer |
|
|
 |
Australian pharmaceutical grade tea tree oil |
|
|
 |
Microcrystalline cellulose M102CG (medicinal excipients) |
|
|
 |
Methoxy polyethylene glycol-polylactic acid block copolymer (medicinal excipient) |
|
|
 |
Talc powder (medicinal excipients) |
|
|
 |
Film coating premix (medicinal excipients) |
|
|
 |
Carboxymethylcellulose calcium (medicinal excipients) |
|
|
 |
Ethanol (anhydrous) (medicinal excipients) |
|
C2H6O |
 |
Sugar coating premix (medicinal excipients) |
|
|
 |
Powdered cellulose (medicinal excipients) |
|
|
 |
Sucrose core (medicinal excipients) |
|
|
 |
Anhydrous sodium sulfite (medicinal excipients) |
|
|
 |
Hydrochloric acid (medicinal excipients) |
|
|
 |
Film coated premixed adjunct (enteric soluble) (medicinal excipients) |
|
|
 |
West yellow gum (medicinal excipients) |
|
|
 |
Egg yolk lecithin (for injection) (medicinal excipients) |
|
|
 |
Ethanol (99.5%) (medicinal excipients) |
|
C2H6O |
 |
Yellow Vaseline (medicinal excipients) |
|
|
 |
Hydroxypropyl beta-cyclodextrin (for oral use) (medicinal excipients) |
|
|
 |
Polyethylene glycol monomethyl etherified polylactic acid (medicinal excipients) |
|
|
 |
Anhydrous lactose (medicinal excipients) |
|
|
 |
Microcrystalline wax (medicinal excipients) |
|
|
 |
Xylitol (medicinal excipients) |
|
|
 |
Octadecyl alcohol (medicinal excipients) |
|
|
 |
Distearylphosphatidylcholine (medicinal excipient) |
|
|
 |
Triacetin (triacetate glyceride) (medicinal excipients) |
|
|
 |
Refined corn oil (medicinal excipients) |
|
|
 |
Methacrylic acid copolymer type C (medicinal excipients) |
|
|
 |
Acetone (medicinal excipients) |
|
|
 |
Succinic acid (oral) (medicinal excipients) |
|
|
 |
Dipalmitoylphosphatidylcholine (for injection) (medicinal excipients) |
|
|
 |
Methoxy polyethylene glycol-poly(D,L-lactic acid) (for injection) (medicinal excipients) |
|
|
 |
Water based castor oil |
|
|
 |
beta-Alanine, N-coco alkyl derivs., sodium salts |
68608-68-4 |
unspecified |
 |
Starch Pharm |
|
|
 |
Barm pharm grade |
|
|
 |
Medicinal corn oil |
|
|
 |
MANDRAGORA OFFICINARUM ROOT EXTRACT |
|
|
 |
Sucrose core (medicinal excipients) |
|
|
 |
PolyacrylicResin |
|
|
 |
Pharmaceutical grade Cabo 941 |
|
|
 |
Medicinal potassium acetate |
|
|
 |
High purity medicinal solid sodium methoxide |
|
|
 |
Pharmaceutical grade potassium chloride |
|
|
 |
Medicinal adsorption alumina |
|
|
 |
Poloxamer 407 (pharmaceutical excipient) |
|
|
 |
medical PU foam with perfume |
|
|
 |
humid medicinal materials |
|
|
 |
Cellulose acetate succinate |
|
|
 |
Pharmaceutical grade maltodextrin |
|
|
 |
Medicinal calcium lactate |
|
|