| Structure |
Chemical Name |
CAS |
MF |
 |
Pharmaceutical grade polyethylene glycol |
|
|
 |
salicylic acid inclusion complex |
|
|
 |
Pharmaceutical grade polyethylene glycol 6000 |
|
|
 |
Oseltamivir Diastereomer I |
|
|
 |
CORN STARCH PHARMACEUTICAL GRADE |
|
|
 |
Vitamin E (DL-Α-tocopherol) (medicinal excipients) |
|
|
 |
99.7% glycerol (medicinal excipients) |
|
|
 |
Egg yolk lecithin (medicinal excipients) |
|
|
 |
Zinc oxide (medicinal excipients) |
|
|
 |
Cross-linked povidone (medicinal excipients) |
|
|
 |
Wheat starch (medicinal excipients) |
|
|
 |
Urea (medicinal excipients) |
|
|
 |
Tannic acid (medicinal excipients) |
|
|
 |
Ammonium sulfate (medicinal excipients) |
|
|
 |
Medicinal hypromellose hollow capsule |
|
|
 |
Mannitol (medicinal excipients) |
|
|
 |
Ethanol (medicinal 95%) (medicinal excipients) |
|
|
 |
Azelaic acid (medicinal excipients) |
|
|
 |
Pharmaceutical film coated premixed adjunct (enteric soluble) (medicinal excipients) |
|
|
 |
Polyacrylic resin latex (gastric collapse) (medicinal excipients) |
|
|
 |
Tetrafluoroethane (internal use) (medicinal excipients) |
|
|
 |
Light magnesium oxide (medicinal excipients) |
|
|
 |
Medicinal hydroxypropyl starch hollow capsule |
|
|
 |
Absolute ethanol (medicinal excipients) |
|
|
 |
Calcium diphosphate (medicinal excipients) |
|
|
 |
Methylparaben (medicinal excipients) |
|
|
 |
L-malic acid (medicinal excipients) |
|
|
 |
Polycarbophil (medicinal excipients) |
|
|
 |
Gluconolactone (medicinal excipients) |
|
|
 |
Red iron oxide (medicinal excipients) |
|
|
 |
Stevioside (medicinal excipients) |
|
|
 |
Medicinal ink (yellow) (medicinal excipients) |
|
|
 |
BEESWAX |
8006-40-4 |
|
 |
783 type medicinal activated carbon |
|
|
 |
Pharmaceutical grade Cabo 934 |
|
|
 |
Medicinal Xylitol |
|
|
 |
Total ionic strength regulator PH5.0-5.5 |
|
|
 |
magnesium carbonate for medicanaln |
|
CMgO3 |
 |
Pharmaceutical grade polyacrylic resin No. 3 |
|
|
 |
Needle, and a pharmaceutically acceptable activated reagent |
|
|
 |
Pharmaceutical film coating premixes |
|
|
 |
Pharmaceutical sodium sulfite (anhydrous) |
|
|
 |
Gelatin hollow capsule |
|
|
 |
Medicinal dextrin |
|
|
 |
Magnesium stearate (medicinal excipients) |
|
|
 |
Tartaric acid (medicinal excipients) |
|
|
 |
Dimethicone (medicinal excipients) |
|
|
 |
Methanesulfonic acid (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 4000 |
|
|
 |
Pharmaceutical grade povidone K30 |
|
|
 |
Light liquid paraffin (medicinal excipients) |
|
|
 |
Sodium thiosulfate (medicinal excipients) |
|
|
 |
DL-tartaric acid (medicinal excipients) |
|
|
 |
Anhydrous sodium acetate (medicinal excipients) |
|
|
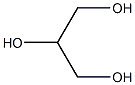 |
Glycerin (for injection) (medicinal excipients) |
|
C3H8O3 |
 |
Magnesium oxide (oral) (medicinal excipients) |
|
|
 |
Ethyl cellulose (medicinal excipients) |
|
|
 |
Ethyl cellulose aqueous dispersion (medicinal excipients) |
|
|
 |
White sugar (medicinal excipients) |
|
|
 |
Polyacrylic resin latex solution for coating premix (medicinal excipients) |
|
|
 |
Pharmaceutical grade povidone K90 |
|
|
 |
Tartaric acid pellet core (medicinal excipient) |
|
C4H6O6 |
 |
Hexadecanol (medicinal excipient) |
|
|
 |
Propylene glycol (medicinal excipients) |
|
|
 |
Medicinal enteric-soluble hypromellose hollow capsule |
|
|
 |
Polyvinyl alcohol (medicinal excipients) |
|
|
 |
Carnitine trisodium (medicinal excipients) |
|
|
 |
Carbomer homopolymer type A (medicinal excipients) |
|
|
 |
Succinic acid (medicinal excipients) |
|
|
 |
Sodium oleate (for injection) (medicinal excipients) |
|
C18H33NaO2 |
 |
Medicinal hollow capsule |
|
|
 |
Activated carbon (for injection) (medicinal excipients) |
|
|
 |
Lanolin (medicinal excipients) |
|
|
 |
Acetic acid-ammonium acetate buffer (pH4.8) |
|
|
 |
Beeswax, synthetic |
71243-51-1 |
|
 |
GOMPHRENA OFFICINALIS ROOT EXTRACT |
|
|
 |
Trihydrate medicinal sodium acetate |
|
|
 |
Medicinal sodium benzoate |
|
|
 |
Soybean oil (for injection) (pharmaceutical excipient) |
|
|
 |
CARBOMER 980 |
139637-85-7 |
|
 |
Medicinal silica gel dryer |
|
|
 |
Ferric chloride,medicinal |
|
FeCl3·6H20 |
 |
Polyester bottle (medicinal) |
|
|
 |
Pharmaceutical grade poloxamer 188F68 |
|
|
 |
Pharmaceutical grade sodium carboxymethyl cellulose |
|
|
 |
Rhubarb (medicinal rhubarb) |
|
|
 |
Medicinal hydrochloric acid |
|
|
 |
Span of S - 80 |
|
|
 |
Medicinal zinc oxide |
|
|
 |
Medicinal yellow beeswax |
|
|
 |
Tocofersolan |
9002-96-4 |
(C2H4O)nC33H54O5 |
 |
Carbomer 934P (medicinal excipients) |
|
|
 |
PRAMIPEXOLE DIMER IMPURITY II |
|
|
 |
Carbomer homopolymer C type 980 NF (medicinal excipient) |
|
|
 |
Dextrin (pharmaceutical excipients) |
|
|
 |
Sucrose (pharmaceutical excipients) |
|
|
 |
85% glycerin (pharmaceutical excipient) |
|
|
 |
Methacrylic acid-ethyl acrylate copolymer and its aqueous dispersion, partially neutralized copolymer (pharmaceutical excipient) |
|
|
 |
Medicinal enteric-coated gelatin hollow capsules |
|
|
 |
Enteric-coated film coating premix (pharmaceutical excipients) |
|
|