| Structure |
Chemical Name |
CAS |
MF |
 |
Cassava starch (pharmaceutical excipient) |
|
|
 |
Potassium chloride (medicinal excipients) |
|
|
 |
Disodium hydrogen phosphate (medicinal excipients) |
|
|
 |
Sodium dihydrogen phosphate (medicinal excipients) |
|
|
 |
Sodium bisulfite (medicinal excipients) |
|
|
 |
Docusate sodium (medicinal excipients) |
|
|
 |
Silica (SYLYSIA 770FCP) (medicinal excipients) |
|
|
 |
Hydroxypropyl cellulose (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 8000 |
|
|
 |
Hydroxypropyl beta-cyclodextrin (medicinal excipients) |
|
|
 |
Polyacrylic resin III (medicinal excipients) |
|
|
 |
1,1,1,2,3,3,3-heptafluoropropane (medicinal excipients) |
|
|
 |
Glyceric acid (medicinal excipients) |
|
|
 |
Beta-cyclodextrin (medicinal excipients) |
|
|
 |
Cetyl alcohol (medicinal excipients) |
|
|
 |
Tricaprylin (medicinal excipients) |
|
|
 |
Xanthan gum (medicinal excipients) |
|
|
 |
Yamanite oleate (Span 80) (medicinal excipients) |
|
|
 |
Polymethacrylic resin latex (medicinal excipients) |
|
|
 |
Disodium edetate (USP) (medicinal excipients) |
|
C10H14N2Na2O8 |
 |
Laurel Yamanashi (Span 20) (medicinal excipients) |
|
|
 |
Sodium stearyl fumarate (medicinal excipients) |
|
|
 |
Calcium diphosphate dihydrate (medicinal excipients) |
|
|
 |
hypochlorous acid |
7790-92-3 |
ClHO |
 |
Glycerides, coco mono-, di- and tri-, hydrogenated |
91744-42-2 |
|
 |
FOMES OFFICINALIS (MUSHROOM) EXTRACT |
|
|
 |
Yellow dextrin |
|
(C6H10O5)x |
 |
Medicinal borax |
|
|
 |
Anhydrous medicinal sodium acetate |
|
|
 |
Pharmaceutical benzoic acid |
|
|
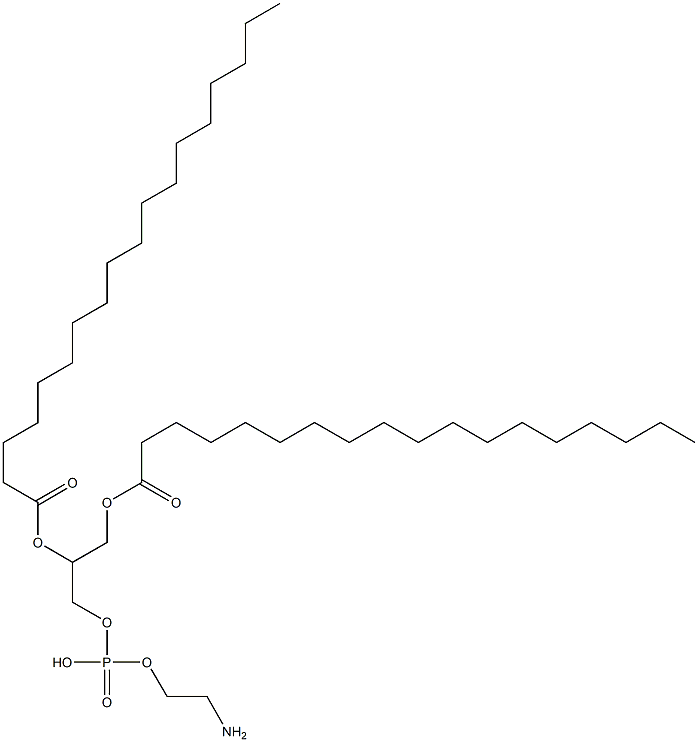 |
1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOETHANOLAMINE |
1069-79-0 |
C41H82NO8P |
 |
20-Epi-16’-decarbomethoxy 19,20-conoduramine |
|
C41H54N4O3 |
 |
Medicinal PVC hard tablets |
|
|
 |
Medicinal calcium sulfate |
|
|
 |
Pharmaceutical ammonium sulfate |
|
|
 |
shuinifapaoji |
|
|
 |
CROSCARMELLOSE SODIUM |
74811-65-7 |
Null |
 |
Pharmaceutical film coating premix (porcine liver color) |
|
|
 |
Glycolide lactide copolymer (7525) (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 1500 |
|
|
 |
Silica (SYLYSIA 770FCP) (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 8000 |
|
|
 |
Film coating premix (stomach soluble type) (medicinal excipients) |
|
|
 |
Protamine sulfate (pharmaceutical excipient) |
|
|
 |
M-cresol (medicinal excipients) |
|
|
 |
1,1,1,2-tetrafluoroethane (medicinal excipients) |
|
|
 |
Silicified microcrystalline cellulose (medicinal excipients) |
|
|
 |
Low-substituted hydroxypropyl cellulose (medicinal excipients) |
|
|
 |
Heptafluoropropane (medicinal excipients) |
|
|
 |
Benzyl alcohol (medicinal excipients) |
|
|
 |
Sodium octanoate (medicinal excipients) |
|
|
 |
Sodium bicarbonate (medicinal excipients) |
|
|
 |
Paraffin wax (SASOLWAX 2697) (medicinal excipients) |
|
|
 |
Sucralose (medicinal excipients) |
|
|
 |
Ethanol (95%) (medicinal excipients) |
|
C2H6O |
 |
Liquid paraffin (medicinal excipients) |
|
|
 |
Plant-derived recombinant human serum albumin (pharmaceutical excipient) |
|
|
 |
Polyammonium methacrylate I (medicinal excipients) |
|
|
 |
Pregelatinized starch (medicinal excipients) |
|
|
 |
Paraffin (medicinal excipients) |
|
|
 |
Medicinal gelatin hollow capsule (ozone sterilization) |
|
|
 |
Ethoxylated ammonium alkyl sulfate (medicinal excipients) |
|
|
 |
Dipalmitoylphosphatidylglycerol (pharmaceutical excipient) |
|
|
 |
Hydrogenated castor oil (medicinal excipients) |
|
|
 |
Ethyl oleate (medicinal excipients) |
|
|
 |
Carbomer homopolymer C type 980 NF (medicinal excipient) |
|
|
 |
Dichloromethane (medicinal excipients) |
|
|
 |
Yellow iron oxide (medicinal excipients) |
|
|
 |
Hydrogenated soybean phosphatidylcholine (pharmaceutical excipient) |
|
|
 |
Medicinal ink (red) (medicinal excipients) |
|
|
 |
ACETIC ACID-AMMONIUM ACETATE |
|
C4H11NO4 |
 |
Pregelled starch |
|
|