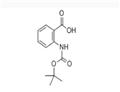
- BOC-2-ABZ-OH
-
- $1.00 / 1KG
-
2019-07-06
- CAS:68790-38-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20kg
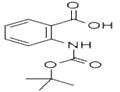
- BOC-2-ABZ-OH
-
- $1.00 / 1KG
-
2019-07-06
- CAS:68790-38-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1000KG
|
| | BOC-2-ABZ-OH Basic information |
| Product Name: | BOC-2-ABZ-OH | | Synonyms: | N-tert-Butoxycarbonylanthranilic acid;Boc-2-Abz-OH >=98.0% (T);BOC-AMINOBENZOIC ACID;BOC-ANTHRANILIC ACID;BOC-ANT-OH;Boc-2-aminobenzoic acid≥ 98% (HPLC);2-(BOC-AMINO)BENZOIC ACID;2-(TERT-BUTYLOXYCARBONYLAMINO)-BENZOIC ACID | | CAS: | 68790-38-5 | | MF: | C12H15NO4 | | MW: | 237.25 | | EINECS: | | | Product Categories: | Amino Acids | | Mol File: | 68790-38-5.mol |  |
| | BOC-2-ABZ-OH Chemical Properties |
| Melting point | 153-156 °C (dec.) | | Boiling point | 328.6±25.0 °C(Predicted) | | density | 1.242±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | pka | 3.67±0.36(Predicted) | | Appearance | White to off-white Solid | | BRN | 2848891 | | Major Application | peptide synthesis | | InChI | 1S/C12H15NO4/c1-12(2,3)17-11(16)13-9-7-5-4-6-8(9)10(14)15/h4-7H,1-3H3,(H,13,16)(H,14,15) | | InChIKey | BYGHHEDJDSLEKK-UHFFFAOYSA-N | | SMILES | CC(C)(C)OC(=O)Nc1ccccc1C(O)=O | | CAS DataBase Reference | 68790-38-5 |
| Safety Statements | 22-24/25 | | WGK Germany | 3 | | HazardClass | IRRITANT | | HS Code | 29242990 | | Storage Class | 11 - Combustible Solids |
| | BOC-2-ABZ-OH Usage And Synthesis |
| Chemical Properties | Beige powder | | Uses | peptide synthesis | | reaction suitability | reaction type: Boc solid-phase peptide synthesis | | Synthesis | GENERAL STEPS: A mixed solution of o-aminobenzoic acid (25.0 g, 182 mmol, 1.0 eq.) and 1:1 THF:H2O (364 mL, 0.5 M) was added to a 1 L round bottom flask. The resulting non-homogeneous mixture was adjusted to pH 10 by dropwise addition of 2N NaOH aqueous solution. di-tert-butyl dicarbonate (43.7 g, 200 mmol, 1.1 eq.) was added to the reaction system, and the resulting homogeneous solution was stirred overnight at room temperature. Upon completion of the reaction, THF was removed by rotary evaporation.Subsequently, the remaining aqueous solution was adjusted to pH 4 by dropwise addition of 15% aqueous citric acid to the remaining aqueous solution so that 2-(N-tert-butoxycarbonylamino)benzoic acid precipitated as a white crystalline solid. The product was collected by vacuum filtration and dried in a vacuum oven to give a white crystalline solid (36.0 g, 88% yield). | | References | [1] Chemistry Letters, 2010, vol. 39, # 11, p. 1127 - 1129
[2] Tetrahedron Letters, 2010, vol. 51, # 29, p. 3855 - 3858
[3] Tetrahedron Letters, 2006, vol. 47, # 38, p. 6739 - 6742
[4] Patent: WO2008/79719, 2008, A1. Location in patent: Page/Page column 51-52
[5] Patent: WO2007/120339, 2007, A1. Location in patent: Page/Page column 41-42 |
| | BOC-2-ABZ-OH Preparation Products And Raw materials |
|