- Diisopropyl D-tartrate
-

- $40.00 / 1kg
-
2025-06-20
- CAS:13811-71-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
|
| | (2S,3S)(-)-Dihydroxybutane-1,4-dioic acid diethyl ester Basic information |
| Product Name: | (2S,3S)(-)-Dihydroxybutane-1,4-dioic acid diethyl ester | | Synonyms: | (-)-Diethyl D-tartrate, Made froM unnatural tartaric acid, 99% 25ML;(R,R)-(+)-Weinsaeure-diethylester;(-)-Diethyl (2S,3S)-2,3-dihydroxybutane-1,4-dioate, (-)-Diethyl (2S,3S)-2,3-dihydroxysuccinate;D-(-)-TARTARIC ACID DIETHYL ESTER;(2S,3S)-(-)-DIETHYL TARTRATE;(2S,3S)(-)-DIHYDROXYBUTANE-1,4-DIOIC ACID DIETHYL ESTER;diethyl [S-(R*,R*)]-tartrate;D-tartrate | | CAS: | 13811-71-7 | | MF: | C8H14O6 | | MW: | 206.19 | | EINECS: | 237-458-8 | | Product Categories: | chiral;Chiral Compounds;Asymmetric Synthesis;Chiral Building Blocks;Esters (Chiral);Synthetic Organic Chemistry;CHIRAL CHEMICALS;Hydroxy Acids & Deriv.;Chiral Compound;Chiral drugs;bc0001 | | Mol File: | 13811-71-7.mol | 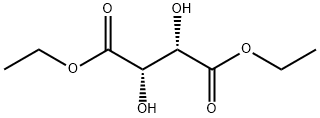 |
| | (2S,3S)(-)-Dihydroxybutane-1,4-dioic acid diethyl ester Chemical Properties |
| Melting point | 17 °C | | alpha | -9 º (neat) | | Boiling point | 162 °C/19 mmHg (lit.) | | density | 1.205 g/mL at 20 °C (lit.) | | refractive index | n20/D 1.446(lit.) | | Fp | 200 °F | | storage temp. | Store below +30°C. | | solubility | Chloroform (Slightly), Water (Slightly) | | form | Viscous Liquid | | pka | 11.61±0.20(Predicted) | | Specific Gravity | 1.21 | | color | Clear colorless | | Optical Rotation | [α]23/D 8.5°, neat | | Water Solubility | insoluble | | Merck | 14,3855 | | BRN | 1727143 | | InChI | 1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m0/s1 | | InChIKey | YSAVZVORKRDODB-WDSKDSINSA-N | | SMILES | CCOC(=O)[C@@H](O)[C@H](O)C(=O)OCC | | CAS DataBase Reference | 13811-71-7(CAS DataBase Reference) | | NIST Chemistry Reference | Butanedioic acid, 2,3-dihydroxy-, diethyl ester, [S-(R*,R*)]-(13811-71-7) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 24/25-36-26 | | WGK Germany | 3 | | Hazard Note | Irritant | | HS Code | 29181300 | | Storage Class | 10 - Combustible liquids |
| | (2S,3S)(-)-Dihydroxybutane-1,4-dioic acid diethyl ester Usage And Synthesis |
| Chemical Properties | Colorless to light yellow liqui | | Uses | Diethyl D(-)-tartrate is mainly used in chiral pharmaceuticals, chiral intermediates and chiral catalysts. It can be used with titanium in enolic asymmetric epoxidization. The compound d-alpha-tocopherol is the principal compound present in natural vitamin E sources. At present, d-alpha-tocopherol and other tocopherol derivatives are used in pharmaceuticals, foods and animal feeds. It is used as a food additive. | | Synthesis | [Refining Procedure No. 1]: In a reactor equipped with a thermometer and a stirrer, 60.0 g of D-tartaric acid (containing 0.20% malic acid and 0.07% fumaric acid) and 36.0 g of ethanol were added, and stirred for 2 hr. at 10° C. The reaction was completed by filtration. After completion of the reaction, 48.1 g of D-tartaric acid was recovered by filtration. [Method 1]: [1st refining step] Dried D-tartaric acid was mixed with 28.8 g of ethanol, 1.9 g of 35% hydrochloric acid was added, and the reaction was carried out at 80°C. Subsequently, the same amount of ethanol and 35% hydrochloric acid was added again at 70 °C, and the reaction was concentrated. The moisture content was confirmed to be 0.1% by weight, and the first esterification reaction solution was obtained. [Method 2]: Ethanol was added to the first esterification reaction solution for stirring, and then 11.4 g of thionyl chloride was added, and the reaction was carried out at 30-40 °C. The reaction was concentrated after completion to obtain the second esterification reaction solution. [Method 3]: Sodium bicarbonate was added to the second esterification reaction solution, and the filtrate was filtered after the reaction to obtain the filtrate (diethyl D-tartrate, 97% yield). Finally, diethyl D-(-)-tartaric acid with an optical purity of 99.8% ee was isolated by film distillation (hot medium temperature 145 °C) under reduced pressure, with diethyl malate and diethyl fumarate contents of less than 0.01%. In addition, the contents of diethyl sulfite and monoethyl tartrate were less than 0.01% and 0.02%, respectively. | | Purification Methods | Distil the esters under high vacuum and store them under vacuum or in an inert atmosphere in a desiccator in round bottomed flasks equiped with a vacuum stopcock. They have also been distilled by Kügelrohr distillation and/or by 'wiped-film' molecular distillation. They are slightly soluble in H2O but miscible with EtOH and Et2O. [Gao et al. J Am Chem Soc 109 5770 (5771) 1987, IR: Pristera Anal Chem 25 844 1953, Beilstein 3 III 1025 for D-(-), 3 IV 1232 for L(+).] | | References | [1] Tetrahedron, 2007, vol. 63, # 27, p. 6346 - 6357
[2] Tetrahedron Asymmetry, 2011, vol. 22, # 3, p. 257 - 263
[3] RSC Advances, 2014, vol. 4, # 90, p. 48827 - 48835
[4] RSC Advances, 2013, vol. 3, # 43, p. 20298 - 20307
[5] Patent: CN104003883, 2016, B. Location in patent: Paragraph 0165-0170 |
| | (2S,3S)(-)-Dihydroxybutane-1,4-dioic acid diethyl ester Preparation Products And Raw materials |
|