- Boc-L-Glu(OMe)-OH
-
- $0.00/ kg
-
2025-10-31
- CAS:45214-91-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T+
- Boc-Glu(OMe)-OH
-

- $0.00 / 25Kg/Drum
-
2025-10-31
- CAS:45214-91-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 1000kg
- Boc-Glu(Ome)-OH
-

- $0.00 / 1KG
-
2025-04-04
- CAS:45214-91-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1Ton
|
| | BOC-GLU(OME)-OH Basic information |
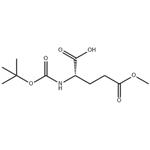
| Product Name: | BOC-GLU(OME)-OH | | Synonyms: | BOC-GLU(OME)-OH;BOC-L-GLUTAMIC ACID GAMMA-METHYL ESTER;N-ALPHA-T-BUTOXYCARBONYL-L-GLUTAMIC ACID GAMMA-METHYL ESTER;BOC-L-GLUTAMIC ACID Y-METHYLESTER;N-ALPHA-BOC-L-GLUTAMIC ACID GAMMA-METHYL ESTER;N-Boc-L-glutamic acid-5-methylester;(S)-2-(tert-butoxycarbonylamino)-5-methoxy-5-oxopentanoic acid;Boc-Glu(OMe)-OH Boc-L-glutaMic acid γ-Methyl ester | | CAS: | 45214-91-3 | | MF: | C11H19NO6 | | MW: | 261.27 | | EINECS: | | | Product Categories: | | | Mol File: | 45214-91-3.mol |  |
| | BOC-GLU(OME)-OH Chemical Properties |
| Melting point | 74-77 °C | | Boiling point | 428.4±40.0 °C(Predicted) | | density | 1.182±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | Chloroform (Sparingly), Methanol (Slightly) | | form | Solid | | pka | 3.82±0.10(Predicted) | | color | White | | InChI | InChI=1S/C11H19NO6/c1-11(2,3)18-10(16)12-7(9(14)15)5-6-8(13)17-4/h7H,5-6H2,1-4H3,(H,12,16)(H,14,15)/t7-/m0/s1 | | InChIKey | OHYMUFVCRVPMEY-ZETCQYMHSA-N | | SMILES | C(O)(=O)[C@H](CCC(OC)=O)NC(OC(C)(C)C)=O |
| | BOC-GLU(OME)-OH Usage And Synthesis |
| Chemical Properties | Boc-L-glutamic acid-5-methyl ester is an amino acid derivative, the molecule contains both acidic and basic groups, therefore, the amino acid can react with stronger acids as well as stronger bases to produce stable salts, with the characteristics of amphoteric compounds, which are mainly used for the preparation of compound amino acid infusion in medicine, and also used for the synthesis of peptide drugs. | | Uses | N-Boc-L-glutamic Acid 5-Methyl Ester is an intermediate used in the synthesis of Isodesmosine Chloride Hydrate (Synthetic) (I815051), which is a component of elastin and also it is extremely hygroscopic and must be stored over a desiccant such as silica gel. | | Synthesis | In a round-bottomed flask, triethylamine (188 g, 1.86 mol, 1.0 eq.) was slowly added dropwise to a stirred mixed solution containing di-tert-butyl dicarbonate (162 g, 0.744 mol, 1.2 eq.) and L-glutamic acid-5-methyl ester (100 g, 0.62 mol, 1.0 eq.) in a solvent consisting of a mixture of water (500 mL) and 1,4-dioxane (500 mL) in a solvent mixture of water (500 mL) and 1,4-dioxane (500 mL). The reaction mixture was stirred continuously for 18 hours at room temperature. Upon completion of the reaction, the reaction solution was extracted with methyl tert-butyl ether (MTBE, 500 mL x 2). The aqueous phase was cooled in an ice bath and the pH was carefully adjusted to 3 by slowly adding 10% citric acid solution.Subsequently, the carbamate product in the aqueous phase was extracted with ethyl acetate (500 mL × 2). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford (S)-2-((tert-butoxycarbonyl)amino)-5-methoxy-5-oxopentanoic acid (Compound A4-2) as a clear viscous oil (180 g, 100% yield). Mass spectrometry (MS-ESI) analysis showed [M + 1]+ m/z 262.1. | | References | [1] Patent: WO2018/67422, 2018, A1. Location in patent: Page/Page column 47
[2] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 13, p. 3793 - 3797
[3] Patent: US2010/184805, 2010, A1. Location in patent: Page/Page column 33-34
[4] Chemical Communications, 2001, # 8, p. 717 - 718
[5] Patent: US2016/16890, 2016, A1. Location in patent: Paragraph 0165-0166 |
| | BOC-GLU(OME)-OH Preparation Products And Raw materials |
|