- FMOC-SER-OME
-
- $1.00 / 1KG
-
2019-07-06
- CAS:82911-78-2
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 20kg
|
| | FMOC-SER-OME Basic information |
| Product Name: | FMOC-SER-OME | | Synonyms: | (S)-Methyl 2-((((9H-fluoren-9-yl)Methoxy)carbonyl)aMino)-3-hydroxypropanoate;FMOC-L-SERINE METHYL ESTER;FMOC-SERINE-OME;FMOC-SER-OME;N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-SERINE METHYL ESTER;Fmoc-L-Ser-OMe;(S)-Fmoc-2-Amino-3-hydroxypropionic acid methyl ester;Fmoc-L-serine methyl ester≥ 99.5% (HPLC) | | CAS: | 82911-78-2 | | MF: | C19H19NO5 | | MW: | 341.36 | | EINECS: | | | Product Categories: | | | Mol File: | 82911-78-2.mol | 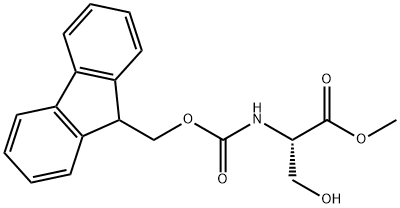 |
| | FMOC-SER-OME Chemical Properties |
| Boiling point | 579.4±45.0 °C(Predicted) | | density | 1.285±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | form | Solid | | pka | 10.07±0.46(Predicted) | | color | White to off-white |
| | FMOC-SER-OME Usage And Synthesis |
| Chemical Properties | White to off-white powder | | Uses | Fmoc-Ser-OMe (Fmoc-L-Ser-OMe) is a hydroxylated L-amino acid protected with a 9-fluorenylmethyloxycarbonyl (Fmoc) group. Fmoc-Ser-OMe involves in chlorophyll–amino acid conjugates synthesis, and acts as a chromo/fluorophores modified protein and emits visible to near-infrared lights efficiently. Fmoc-Ser-OMe glycosylates and produces small mucin-related Olinked glycopeptides, as an alcohol acceptor[1][2]. | | Synthesis | GENERAL STEPS: To a mixed solution of 1,4-dioxane (15 mL) and water (90 mL) containing L-serine methyl ester hydrochloride (10.0 g, 64.3 mmol), sodium bicarbonate (10.8 g, 129 mmol) was added. The reaction mixture was stirred at room temperature for 15 minutes. Subsequently, a solution of 1,4-dioxane (60 mL) of 2,5-dioxopyrrolidin-1-yl 9H-fluoren-9-ylmethyl carbonate (21.7 g, 64.3 mmol) was slowly added to the reaction system, and stirring was continued at room temperature for 14 hours. Upon completion of the reaction, water was added to the reaction mixture and extracted three times with ethyl acetate. The organic layers were combined and washed sequentially with water and saturated brine. The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Ether and heptane were added to the residue and the precipitate was collected by filtration to give fmoc-L-serine methyl ester (22.3 g, yield: quantitative). The product was characterized by 1H-NMR (400 MHz, CDCl3): δ 2.00-2.15 (1H, m), 3.81 (3H, s), 3.89-4.07 (2H, m), 4.20-4.28 (1H, m), 4.39-4.53 (3H, m), 5.63-5.74 (1H, m), 7.29-7.37 (2H , m), 7.38-7.46 (2H, m), 7.55-7.65 (2H, m), 7.74-7.82 (2H, m). | | References | [1] Tamiaki H, et al. Synthesis of chlorophyll-amino acid conjugates as models for modification of proteins with chromo/fluorophores. Bioorg Med Chem. 2014 Feb 15;22(4):1421-8. DOI:10.1016/j.bmc.2013.12.059
[2] K?rkk?inen TS, et al. Iodine-mediated glycosylation en route to mucin-related glyco-aminoacids and glycopeptides. Carbohydr Res. 2008 Jul 21;343(10-11):1830-4. DOI:10.1016/j.carres.2008.03.034 |
| | FMOC-SER-OME Preparation Products And Raw materials |
|