|
|
| | 1,1'-Bis(diphenylphosphino)ferrocene Basic information | | Reaction |
| Product Name: | 1,1'-Bis(diphenylphosphino)ferrocene | | Synonyms: | DPPF;1,1''-BIS(DIPHENYLPHOSPHINO)ERROCENE;1,1''-BIS(DIPHENYLPHOSPHINO)FERROCENE (DPPF);DPPF/1,1''-BIS(DIPHENYLPHOSPHINO) FERROCENE;1,1'-Bis(diphenylphosphiNA)ferrocene;DPPF, (Ferrocene-1,1'-diyl)bis(diphenylphosphine);1,1-(twophenylphosphine)Er Maotie;1,1'-FERROCENEDIYL-BIS(DIPHENYLPHOSPHINE) | | CAS: | 12150-46-8 | | MF: | C34H28FeP210* | | MW: | 554.38 | | EINECS: | 640-119-0 | | Product Categories: | Miscellaneous;pharmacetical;Ligand;Catalysts-Ligands;Classes of Metal Compounds;Fe (Iron) Compounds;Ferrocenes;Metallocenes;Phosphine Ligands;Synthetic Organic Chemistry;Transition Metal Compounds;Boron, Nitrile, Thio,& TM-Cpds;Achiral Phosphine;Aryl Phosphine;Pharmaceutical intermediates;Phosphines;Chelating Agents & Ligands;Catalyst;Aromatics | | Mol File: | 12150-46-8.mol |  |
| | 1,1'-Bis(diphenylphosphino)ferrocene Chemical Properties |
| Melting point | 181-182 °C (dec.)(lit.) | | storage temp. | 2-8°C | | solubility | Chloroform, Ethyl Acetate | | form | crystal | | color | yellow to orange | | Water Solubility | Soluble in chloroform, dichloromethane, alcohol and pentane. Insoluble in water. | | Sensitive | Air Sensitive | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChI | InChI=1S/2C17H14P.Fe/c2*1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;/h2*1-14H; | | InChIKey | HPXNTHKXCYMIJL-UHFFFAOYSA-N | | SMILES | P(C1C=CC=CC=1)(C1=CC=CC=C1)[C]1[CH][CH][CH][CH]1.P(C1=CC=CC=C1)(C1=CC=CC=C1)[C]1[CH][CH][CH][CH]1.[Fe] |^1:13,14,15,16,17,31,32,33,34,35| | | CAS DataBase Reference | 12150-46-8(CAS DataBase Reference) | | NIST Chemistry Reference | 1,1'-Bis(diphenylphosphino)ferrocene(12150-46-8) |
| | 1,1'-Bis(diphenylphosphino)ferrocene Usage And Synthesis |
| Reaction |
- Ligand for Pd-catalyzed cross-coupling.
- Useful ligand for Pd-catalyzed carbon-nitrogen and carbon-oxygen bond forming procedures.
- Ligand for Ni-catalyzed amination of aryl chlorides.
- Ligand for Pd-catalyzed conversion of aryl halides to aryl nitriles.
- Ligand for Ni-catalyzed Suzuki reactions.
- Ni-catalyzed hydroamination of 1,3-dienes.
- Pd-catalyzed hydrocarbonation and hydroamination of 3,3-dihexylcyclopropene.
- Pd-catalyzed γ-arylation of β,γ-unsaturated ketones.
- Ligand for Ru-catalyzed reduction of nitriles to primary amines.
- Ligand for Rh-catalyzed alkyne head-to-tail dimerization.
- Ligand for Rh-catalyzed cross-coupling
- Ligand for Rh-catalyzed olefin isomerization
- Ligand for Ni or Rh-catalyzed borylation
- Ligand for regioselective Pd-catalyzed hydrophosphinylation of terminal alkynes to form branched alkenes.
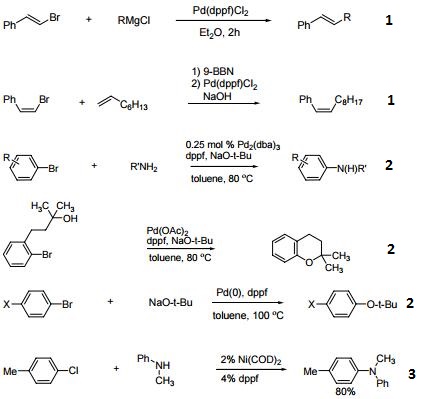
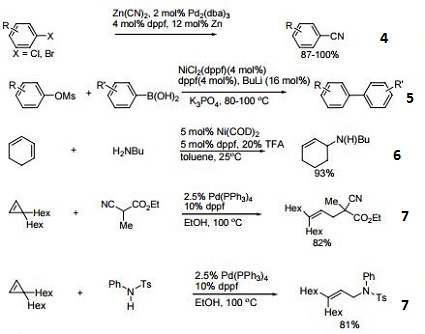
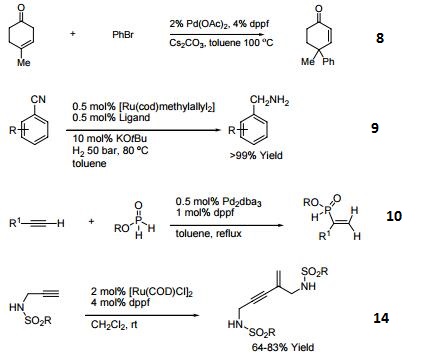
| | Chemical Properties | 1,1'-Bis(diphenylphosphino)ferrocene is deep yellow crystalline powder | | Uses | 1,1'-Bis(diphenylphosphino)ferrocene used coordination compound in synthesis, readily forms complexes with various metals, i.e. when reacting with the acetonitrile or benzonitrile complexes of PdCl2 it forms (dppf)PdCl2, which i s a popular reagent for palladium-catalyzed coupling reactions. | | Uses | 1,1'-Bis(diphenylphosphino)ferrocene acts as a ligand in homogeneous catalysis. It is used as a ligand for ruthenium-catalyzed greener amine synthesis from amines and alcohols by hydrogen-borrowing. It is also employed as a ligand for Buchwald-Hartwig cross coupling. Further, it is used in the synthesis of coordination compound as well as the formation of complexes with various metals such as palladium chloride. In addition to this, it serves as a reagent for palladium-catalyzed coupling reactions and also plays an important role in Suzuki reaction. | | General Description | Novel functionalized furan derivatives were prepared via Pd-phosphine sequential C-C and C-O bond formation. | | reaction suitability | reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Carbonylations
reagent type: ligand
reaction type: Ene Reaction
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Tsuji-Trost Reaction | | Synthesis | The general procedure for the synthesis of 1,1'-bis(diphenylphosphino)ferrocene (dppf) from the compound (CAS: 405164-68-3) is as follows:
Example 4: 100 mg (0.171 mmol) of 1,1'-bis(diphenoxyphosphino)ferrocene was reacted with 4 mg (16 μmol) of I? and 170 μL (0.68 mmol) of tributylphosphine in 1 mL of acetonitrile/THF (1:1, v/v) mixed solvent. The reaction mixture was stirred at room temperature for 10 min under nitrogen protection, followed by quenching the reaction with 100 μL of H?O. The reaction was then quenched with 10 mL of ethyl acetate. The reaction mixture was diluted with 10 mL of ethyl acetate and washed three times (5 mL each) with saturated aqueous NaHCO?solution. The organic phase was dried with Na?SO?, filtered and concentrated under reduced pressure. The resulting residue was recrystallized with ethanol, the crystals were collected by filtration, washed and dried under vacuum to give 89 mg (0.160 mmol, 94% yield) of 1,1'-bis(diphenylphosphino)ferrocene (dppf). | | Purification Methods | Wash it with distilled H2O and dry it in a vacuum. Dissolve it in ca 5 parts of hot dioxane and cool to give orange crystals m 181-183o. Recrystallisation from *C6H6/heptane (1:2) gives a product with m 183-184o. [Bishop et al. J Organomet Chem 27 241 1971.] | | References | [1] Patent: WO2011/123037, 2011, A1. Location in patent: Page/Page column 23 |
| | 1,1'-Bis(diphenylphosphino)ferrocene Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Ethyl acetate-->Tetrahydrofuran-->Dichloromethane-->n-Butyllithium-->N,N,N',N'-Tetramethylethylenediamine-->Ferrocene-->Dicyclopentadiene-->Ferrous chloride-->Chlorodiphenylphosphine-->Lithium, [1-(diphenylphosphino)-2,4-cyclopentadien-1-yl]--->Diphenylphosphinoferrocene-->1,1'-bis(diphenylphosphinyl)-Ferrocene-->4,4'-Bis(t-butyl)-1,1',2,2'-tetrakis(diphenylphosphino)ferrocene, 98% HiersoPHOS-5-->DIMETHYLPHENYLPHOSPHINE-->Sodium thiocyanate-->Sodium diethyldithiocarbamate-->Phenyllithium | | Preparation Products | [1,1'-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)-->N,N'-Di-1-naphthyl-N,N'-diphenylbenzidine (NPB)-->1,1'-Bis(diphenylphosphino)ferrocene-palladium(II)dichloride dichloromethane complex-->[1,1'-Bis(diphenylphosphino)ferrocene]dichloronickel(II) |
|